Research Highlights
A Small Population of Immune Cells Is Key For Cancer Immunotherapy
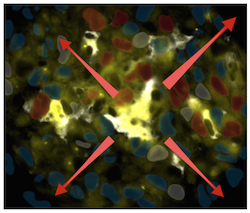
The anti-PD-1 immune checkpoint blocker can induce sustained clinical responses in some patients. This drug is used to release the “brakes” on T cells but how it functions in vivo remains incompletely understood. In a study published in Immunity, the Pittet lab at the MGH Center for Systems Biology uncovers that effective antitumor responses to anti-PD-1 therapy requires a subset of tumor-infiltrating dendritic cells, which produce interleukin 12 (IL-12) and apply “gas” to fuel the antitumor reaction. The findings may lead to new treatment strategies that benefit more patients. [Image: KA-POW! A dendritic cell (yellow) interacts with antitumor T cells (blue) to get rid of cancer.]
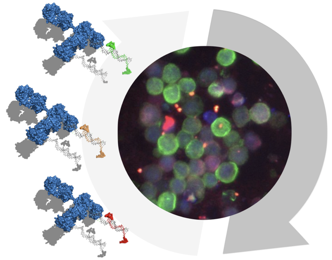
Antibody-DNA conjugates bring patient biopsies to light
Modern oncology relies on molecular assessments of tumor tissue to develop new therapeutic combinations and select optimal treatments for individual patients. Conventional approaches to cellular fluorescence imaging allow for visualization of just 3-4 proteins at a time, limiting the amount of information we can gain from precious patient biopsy samples. In a new study published in Nature Communications, researchers at CSB developed an approach that uses antibody-DNA conjugates to efficiently “cycle” through the detection and quantification of multiple proteins of interest, dramatically increasing the number of pathways/targets that can be imaged from a single biopsy. This technique allows scientists/physicians to directly visualize complex protein signatures in the biopsied cells and has important implications for understanding how drug therapies affect patients' individual tumors.
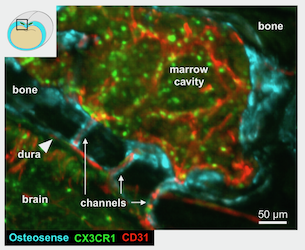
Shortcut to the brain
White blood cells are our key defenders against infection, however if oversupplied, they may turn against us. Neutrophils are made in the bone marrow where they arise from hematopoietic stem cells. The bone marrow is distributed over many bones in our bodies, and the current thinking implies that the supply of leukocytes distributes evenly throughout the body. In work published in Nature Neuroscience, we describe that the skull marrow assumes a special role in inflammatory diseases of the CNS. Its proximity to the brain leads to a preferential supply of neutrophils. We detected a previously unknown shortcut that neutrophils use on their way from skull marrow cavities towards the central nervous system. Rather than traveling through the general blood circulation, leukocytes produced in skull bone marrow migrate through channels that directly connect the skull marrow with the meninges the brain is wrapped in. The channels exist in mice and humans.

Artificial Intelli-sense
Automating cellular diagnostics could have far reaching impact in healthcare. Cells - often obtained by aspirations, biopsies, swabs or through body fluids - typically require sophisticated instrumentation and time consuming experts analysis to provide diagnoses. The CSB engineering team has now developed a highly sensitive platform powered by digital imaging and artificial intelligence to automate such painstaking analyses. Moreover this platform is affordable and portable, thus uniquely suited for point-of-care diagnostics in low and middle income countries (LMIC). A recent study published in Nature Biomedical Engineering highlights the first clinical trial for lymphoma diagnostics.

Re-educating Tumors
Tumor-associated macrophages (TAM) are abundant in many cancers and often display an immune-suppressive phenotype that promotes tumor growth and resistance to treatment. Researchers at CSB have now developed a TAM targeted nanoparticle loaded with a toll-like receptor agonist which re-programs TAMs to support the immune-system’s fight against cancer. As a monotherapy, administration of the drug-loaded nanoparticle led to efficient drug delivery to TAMs, re-programming of TAMs to an immune-supportive phenotype, and controlled tumor growth. Importantly, the strategy worked synergistically in combination with checkpoint therapy (anti-PD1), dramatically improving response rates even in tumors resistant to treatment by anti-PD1 alone. These findings demonstrate the ability of rationally engineered drug–nanoparticle combinations to efficiently modulate TAMs to better sensitize the tumor microenvironment to standard checkpoint therapies.

Bones and Neutrophils Control Lung Cancer
Tumors are often infiltrated by diverse immune cell types, some of which remain largely unexplored. In a study published in Science, the Pittet lab at the MGH Center for Systems Biology uncovers a new type of neutrophil that promotes lung cancer. The production of these neutrophils involves an unexpected remote crosstalk between tumors and bones: lung tumors remotely activate osteoblasts; in turn, those bone cells shape immunity by supplying tumors with cancer-promoting neutrophils. The findings open new avenues for cancer immunotherapy. (Image from Wikipedia)
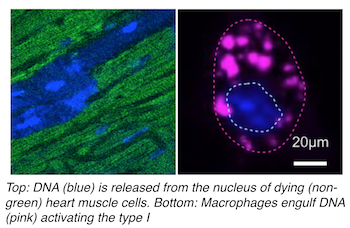
Immune cells attend a heart attack masquerade
Hearts attacks, result from occlusion of coronary arteries, which starves heart muscle cells of oxygen-rich blood and causes them to die. Immune cells respond by entering the dead tissue, clearing cell debris, and stabilizing the heart wall via fibrosis and repair. In their Nature Medicine report, CSB investigators describe the surprising finding that dying cell DNA mimics a virus, which causes immune cells to turn on antiviral programs after a heart attack even though there is no viral infection.

Keychain detector catches food allergens before it’s too late
More than 50 million Americans display food reactions. Each year there are an estimated over 20,000 food allergy-related emergency department visits in the United States, including 90,000 cases of anaphylaxis. The best way to manage food allergy is to avoid products that contain allergen. But avoidance isn't always possible because food can be mislabeled or cross-contaminated.
Meet iEAT (integrated Exogenous Antigen Testing) a $40 portable allergen-detection system that consists of a disposable kit to extract allergens from food and an electronic keychain analyzer for allergen detection. In less than 10 minutes, the iEAT completes food analyses and sends the results to a cloud server. The prototype was used to detect five model allergens from wheat, peanuts, hazelnuts, milk and egg white. Testing on food items from local restaurants revealed unexpected findings such as gluten in “gluten-free” dishes and egg protein in beer. The technology is being expanded to detect additional allergens, pesticides and environmental hormones.

What does a catalytic converter have to do with drugs?
A lot, it turns out. Catalytic converters in our cars convert toxic gas emissions into into acceptable ones. A key element is the metal palladium, which catalyses the oxidation of pollutants like carbon monoxide to carbon dioxide. In a recent article in Nature Communications, researchers at CSB have developed a medical version of nano-palladium to enable chemistry to take place inside cells in our body. The discovery allows the administration of harmless prodrugs, which then get specifically activated at sites of cancer.
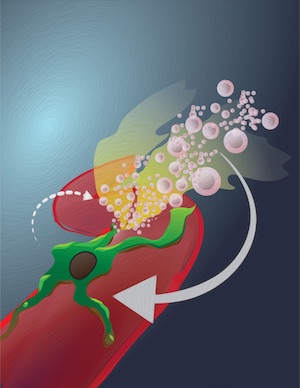
A new trick for macrophages
Macrophages, immune cells in our body, have a long to-do list. They defend us from bacteria, are essential in wound healing, keep the heart beating and perform other vital tasks. In a new twist, these cells are now shown to dramatically accumulate on the outside of cancer microvessels following radiation therapy. There, they elicit dynamic and focally localized bursts of capillary leaks. This in turn enhances drug delivery, especially of nanomaterials. These new insights have implications for the design of next-generation tumor targeted nanomaterials and clinical trials for adjuvant strategies.
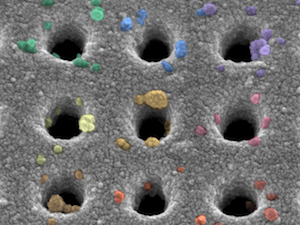
A signature achievement
Pancreatic ductal adenocarcinoma is one of the deadliest types of tumors, in part because it is usually detected at a late stage. To facilitate the diagnosis of this tumor, researchers at CSB have developed a multiplexed nanoplasmonic assay to analyze extracellular vesicles in blood of patients. While some blood biomarkers have previously been proposed, none of them have proven sufficiently accurate in clinical practice. We have now identified a new five-marker signature that yielded the most accurate diagnosis in a large cohort of patient samples.
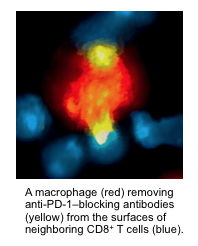
Spotlight (literally) on immunotherapy
Immunotherapy and especially immune checkpoint blockers (ICBs) are revolutionizing how we treat many cancers. Designed to activate the immune system, these drugs can be extraordinarily effective in some patients. But progress has been slowed by our limited understanding of why ICBs work well in some cancers and patients but not in others. Now, Mikael Pittet and colleagues have used molecular imaging to track ICBs in real time and at high resolution within tumors. Their study, published in Science Translational Medicine, uncovers a previously undiscovered mechanism of treatment resistance, which can be overcome with additional chemical modifications.
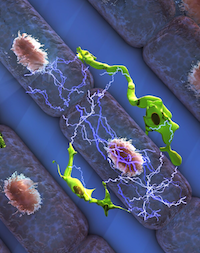
Cardiac macrophages charging ahead
While we knew for a while that the healthy heart contains tissue resident macrophages, these cells’ organ specific functions were unknown. Triggered by a serendipitous finding of ECG abnormalities during a cardiac MRI scan of a mouse after macrophage ablation, a CSB team of investigators now describes previously unknown electrical properties of macrophages. When coupled to myocytes via gap junctions, macrophages depolarize in sync with conducting cells. In a sink-source relationship, electric current flows back and forth between macrophages and cardiomyocytes. Macrophages influence conduction through the atrioventricular node, the electrical connection between the heart’s chambers. When macrophages are manipulated, the flow of electricity slows down, and may even cease altogether. Such a condition requires pacemaker treatment in humans. These surprising findings, published in Cell, jolt the field of electrophysiology and may lead to new therapeutic opportunities for patients with cardiac arrhythmias. The collaborative effort was spearheaded by teams at MGH but also involved investigators at the BWH and in Freiburg, Germany.
Tiny nanoparticles hunt for macrophages
Macrophages are white blood cells that can turn against us in atherosclerosis. Instead of cleaning up tissue as usual, they attack the arterial wall and destroy its architecture. The resulting stoppage of blood flow causes myocardial infarction and stroke. An entire field of research focuses on understanding these cells, and how to stop them from becoming turn coats. Until now, it was not even possible to detect them reliably in patients. In a recent Nature Communications report, a modified polyglucose nanoparticle (18F-Macroflor) was developed for imaging macrophages by PET. Macroflor enriches in cardiac and plaque macrophages, thereby increasing PET signal in murine infarcts and both mouse and rabbit atherosclerotic plaques. This work marks an important step towards a clinical tool to non-invasively monitor macrophage biology in patients. Such an imaging tool can then be used to spot danger spots in several diseases, and test new macrophage-targeted therapeutics while directly watching the cells.
Ingest, Digest, Recycle: Where red blood cells and the iron they contain are recycled.
Iron gives blood its red color. The metal is essential to life, but it can be toxic because of its oxidative properties. Remarkably, we receive relatively little of our daily iron needs through diet. By far the majority of the iron we need is recycled. According to current thinking, as red blood cells age, large phagocytes residing in the spleen capture them, digest the cell structures, and recycle iron. A new paper from CSB published in Nature Medicine shows that most red blood cell disposal actually occurs in the liver, especially when demands for disposal increase (as they do in many physiologic and pathophysiologic situations). Moreover, specialized white blood cells consume old red blood cells in the circulation before migrating to the liver to shuttle iron for storage and new red blood cell production. The process buffers against dangerous fluctuations in iron availability, keeping the body in balance.
Quintuple-target RNAi: hitting five targets is better than just one
Vascular endothelial cells express five adhesion molecules to recruit leukocytes from the blood stream: E- and P-selectin, ICAM-1 and -2, and VCAM-1. In atherosclerosis, activated endothelial cells express high levels of these signals, thus expanding the number of neutrophils and monocytes that migrate from blood into a growing plaque. After myocardial infarction, the adhesion molecule expression increases even further due to higher autonomic nervous activity. A collaborating team of groups at MIT and MGH now used a new class of nanoparticles with high avidity to endothelial cells to decrease endothelial cell adhesion molecule expression. The polymeric nanoparticles made of low-molecular-weight polyamines and lipids were loaded with 5 distinct siRNAs silencing the expression of all adhesion molecules. Multiple gene silencing was enabled by exquisite silencing efficiency after nanoparticle delivery. Hitting five targets at once, the therapy reduced recruitment of leukocytes to atherosclerotic plaques in mice, dampening vascular wall inflammation and making plaques smaller. Furthermore, RNAi decreased migration of leukocytes into infarcted myocardium, improving the recovery after ischemia. Such a strategy may help to prevent reinfarction and heart failure in high-risk patients with acute MI.
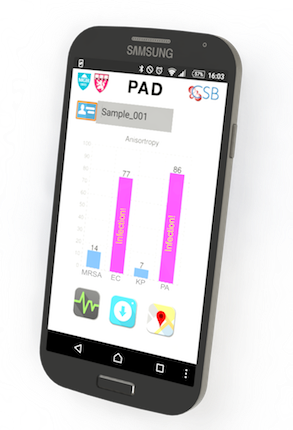
Ultrafast bacterial ID
Rapid and efficient diagnosis of bacterial infection is critical in combating infections, esp. in the hospital setting where drug resistance to antibiotics is on the rise. CSB investigators have developed a new device to shorten the diagnosis of bacterial infection to less than 2 hours. The “PAD” system (short for polarization diagnostics) is a combination of optical read-outs of genetic bacterial information and can ultimately performed in a physician’s office at low cost.
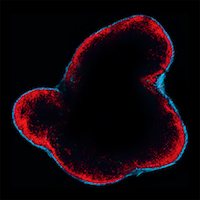
An immune cell that protects against cancer
Macrophages are mostly viewed as tumor-promoting cells. They can infiltrate solid tumors in high numbers, and their presence at the tumor site is often associated with decreased patient survival. However, much less is known about macrophages located outside the tumor stroma. Mikael Pittet and colleagues now show that a population of lymph node macrophages, called subcapsular sinus (SCS) macrophages, unexpectedly protects against melanoma. The study was published in Science on March 17, 2016.
A recipe to improve cancer immunotherapy
Novel immune checkpoint blockade therapies can be extraordinarily effective but may benefit only the minority of patients whose tumors are pre-infiltrated by antitumor immune cells called CD8+ T cells. In a study published in Immunity, the Pittet lab at MGH Center for Systems Biology reports that rationally selected immunogenic chemotherapy can convert tumor microenvironments lacking T cells into ones displaying antitumor T cell immunity. This process makes unresponsive tumors sensitive to immune checkpoint blockade therapies and consequently raises hope to feasibly expand the proportion of human cancers responding to these therapies.
Are nanomedicines right for you?
Nanoparticles promise to deliver toxic chemotherapeutics more safely and efficiently to solid tumors, but clinical responses to such treatments have been mixed: some patients respond extremely well while others do not. Using advanced imaging techniques, researchers at CSB have discovered a way to repurpose FDA-approved magnetic nanoparticles for predicting how effectively nanomedicines can accumulate in tumors. Published in Science Translational Medicine, this “companion diagnostic" approach suggests that clinical imaging can be used to select patients most likely to benefit from the most advanced nanomedicine treatments.
Macrophages act as drug delivery depots of nanomedicines
Solid tumors often contain large numbers of immune cells including macrophages that feed cancer growth and metastasis. CSB researchers discovered that these tumor associated macrophages can be co-opted by nanomaterials to serve as drug depots, gradually delivering chemotherapy to neighboring cancer cells. Driven by new intravital imaging technology and published in Nature Communications, this research presents a new paradigm for therapeutic design and for selecting patients into clinical trials.
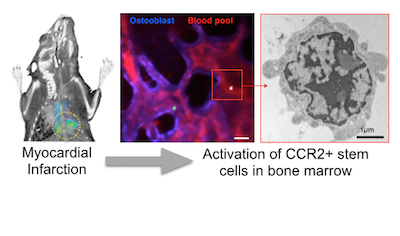
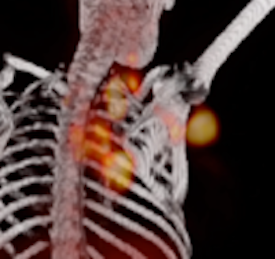
Bone marrow stem cells are alerted to heart attack
Blood cells, including inflammatory monocytes, are made in the bone marrow and ultimately derive from hematopoietic stem cells. Until now it was unknown which bone marrow cells expand in acute myocardial infarction. Recent work published in Cell Stem Cell identified a subpopulation of short-term stem cells as the most upstream activation point after MI. The surface marker CCR2 identifies, in mice and in humans, the cell subset that sit almost at the very top of the hematopoietic tree as particularly responsive to an injury of the heart. The myeloid translocation gene 16 regulates their emergence, and may provide a therapeutic target to dampen leukocyte production that could otherwise jeopardize resolution of inflammatory activity in cardiovascular organs.

Smartphone Sees Cancer
With their ubiquitous presence and superb computation power, smartphones now bring unprecedented opportunities to realize mobile healthcare. Reported in PNAS, CSB researchers have developed a new smartphone-based system, D3 (digital diffraction diagnosis), for on-the-spot molecular detection. This system, complete with a custom App, was used for cervical cancer screen and diagnosing aggressive lymphomas, prevalent cancers in low and middle-income countries.
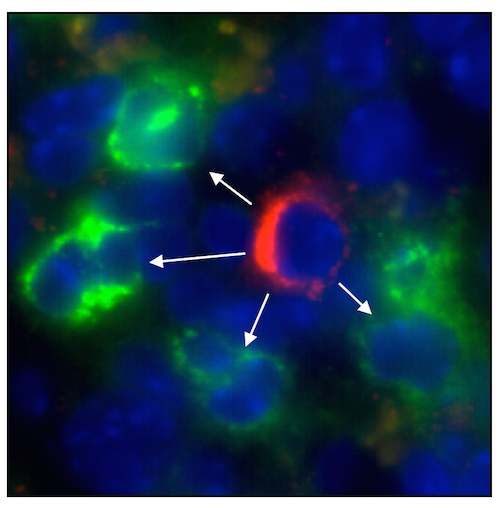
A new lead in solving sepsis
The complication of an infection known as sepsis (or “blood poisoning”) is extremely dangerous, claiming up to half a million lives in the United States every year. A study from the Swirski lab has shown that a growth factor called interleukin-3 (IL-3) amplifies inflammation in sepsis and potentiates septic shock, the most severe form of sepsis. The authors show that IL-3 induces the emergency production of inflammatory monocytes and neutrophils, which are sources of the hallmark cytokines that comprise a lethal cytokine storm. A subset of B-1 B cells, discovered in the Swirski lab and named IRA B cells, are abundant sources of IL-3 in sepsis. Patients diagnosed with sepsis with high IL-3 in their blood die more often than those containing low IL-3. The findings are reported in the March 13th issue of Science.