
Checking out checkpoints
Immune checkpoint blockers (ICBs) are designed to activate the immune system against cancer. These drugs can be extraordinarily effective in some patients but not in others. Now, Mikael Pittet and colleagues have used molecular imaging to track ICBs in real time within tumors. Their study uncovers a mechanism of treatment resistance, which can be overcome with additional chemical modifications. Access to the article, journal cover, video interview with the authors, and press coverage, is included below.
Immune checkpoints are key immune response nodes in which decisions of either inflammation or tolerance are made. Tolerance is promoted by inhibitory checkpoints, which usefully prevent uncontrolled immune responses in the body; however, inhibitory checkpoints can also be co-opted by tumors that make them resist immune attack. For example, although some CD8+ T cells can kill cancer cells, they often express inhibitory checkpoint receptors, such as PD-1, that make them lose their cytotoxic functions, thereby rendering them ineffective against tumors.
Immune checkpoint blockade is used in cancer therapy to inhibit the “brakes” of the immune system. This approach has revolutionized cancer treatment by enabling durable control of previously untreatable cancers. Among immune checkpoint blockers, antibodies blocking the PD-1 pathway are now used to treat a broad range of tumors including melanoma, lung, bladder, head and neck cancer, ovarian and colorectal cancer. These drugs are used with the goal of reactivating tumor-infiltrating CD8+ T cells, which often express PD-1. However, immune checkpoint blockers are only effective in some patients, and little is known about how these drugs behave in tumors and why they work or fail against cancer.
To address these questions, investigators from the Pittet and Weissleder laboratories at Massachusetts General Hospital developed fluorescent versions of PD-1–blocking antibodies that could then be tracked in real-time and at high resolution in living animals using intravital microscopy. “Dynamic observation of anti-PD-1 therapy in the tumor microenvironment provides greater insight into the pharmacologic mechanism,” says first author Sean Arlauckas. “We can discover which cells the therapy interacts with in vivo, and from this information we can map the ‘life cycle’ of the drug at single cell resolution.”
See also this video: Interview with the authors.
The investigators found that PD-1 inhibitors rapidly enter the tumor bed through the vasculature and can then bind to intratumoral CD8+ T cells. “This initial finding was not surprising,” says Christopher Garris, co-first author on the study. “The PD-1 pathway is known to target T cells, so it was reassuring that our imaging studies accorded with prior results.”
However, in following the interactions between CD8+ T cells and PD-1 inhibitors, the team discovered that the drug remained on T cells for only a few minutes. “It became important for us to discover why the CD8+ T cells cannot keep hold of the drug, and also where the drug goes when it separates from T cells,” says Arlauckas. “We started to investigate whether other cells in tumors may capture the PD-1 antibodies, and we found this was indeed the case; as the drug was removed from T cells, it accumulated in other immune cells called macrophages. Tumors frequently contain macrophages, which are known to promote cancer growth, but macrophages don’t express PD-1, so it was surprising to find PD-1 antibodies on these cells,” adds Garris.
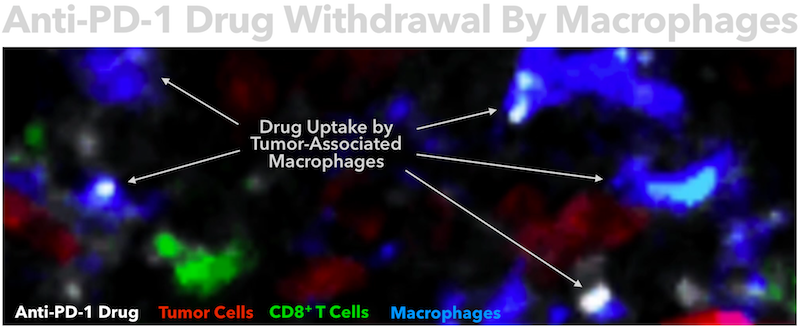
To understand how macrophages remove the drug from T cells, the investigators devised a live-cell in vitro imaging system that allowed them to manipulate T cells and macrophages while tracking them in real time. “Using this system, we confirmed that macrophages strip PD-1 antibodies from the T cell surfaces,” says Arlauckas. “Antibodies contain Fab and Fc regions. The Fab region determines antigen specificity, for example the ability to bind PD-1, while the Fc region can modulate immune cell activity. We found that PD-1 antibody binding to macrophages was not antigen specific, so we hypothesized that the Fc region instead may mediate antibody transfer. In support of this idea, we knew that macrophages express receptors that bind Fc regions of antibodies.” Indeed, selectively blocking Fc receptor binding on macrophages was sufficient to prevent anti-PD-1 transfer, thus demonstrating that Fc receptors are involved in anti-PD-1 uptake.
“Based on these findings, we wondered whether blocking Fc receptors could actually improve immunotherapy,” says Garris. To test this hypothesis, the team co-administered Fc receptor blocking antibodies and anti-PD-1 in tumor-bearing mice and then investigated what happened to the drug and the tumors. “We found that blocking Fc receptors allowed the anti-PD-1 drug to bind CD8+ T cells much longer in vivo. In fact, PD-1–blocking antibodies stayed on T cells for at least several hours, instead of a few minutes as when Fc receptors on macrophages were not blocked. Also, the manipulation dramatically increased treatment efficacy. All the mice tested in this modified therapeutic setting were apparently cured of the disease.”

This work demonstrates that antibody/Fc receptor binding can prove detrimental to some immunotherapies, but also that manipulating these interactions may improve current treatment options. “Considering that checkpoint immunotherapies are all antibody-based, we must factor Fc receptor interactions into their pharmacologic mechanisms. A growing repertoire of monoclonal antibody drugs have entered the market for a wide range of medical conditions, and several approaches can be considered for enhancing these therapies. On our end, we are considering three main approaches: modulating antibodies’ Fc regions, targeting Fc receptors and modulating the cells that express these receptors,” says Mikael Pittet, senior author of the study.
“It’s profound that we can image key readouts of immunotherapy function in real time at single cell resolution,” says Ralph Weissleder, Director of the Center for Systems Biology. “The ability to track pharmacokinetics and pharmacodynamics of biologics in vivo will be important for deciphering drug action mechanisms. We are now on the cusp of leveraging this knowledge into engineering better therapeutics.”

Pittet adds, “Going forward, we are incredibly excited because we have an imaging platform that allows us to assess immune drugs in a way that was not possible before. So far, we have focused on PD-1–blocking antibodies, but many other immune drugs are being, or will soon be, tested in patients, and it is important to better understand how all these drugs behave in vivo. Also, the imaging platform allows us to image different drugs at the same time. This should become handy in better defining next-generation combination treatments.”
Article
In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy by Arlauckas, Garris et al. Science Translational Medicine, 10 May 2017, Vol. 9, Issue 389, eaal3604
Journal Cover
Tug-of-war with anti-PD-1
Video
Interview with the authors
Press coverage
Turmoil in Immuno-oncology – “In the Pipeline”, Science Translational Medicine blog
Light-Fingered Macrophages Caught Red Handed at Checkpoint – GEN News Highlights
Molecular imaging reveals mechanism for resistance to immune checkpoint blockade – Medical Express
Descubren un mecanismo del cáncer para resistir tratamientos inmunitarios – CronicaCica (in spanish)
Macrophages that rapidly remove drugs from T cells may be new treatment target – Eureka Alert
Surface cleaning – Harvard Medical School
Tumor-dwelling immune cells thwart cancer immunotherapy- Medical News Today
Study explains how macrophages block anti-PD-1 mAbs- Biocentury
Competition between immune cells could explain why some immunotherapu drugs fail- BioPortfolio
Competition between immune cells could explain why some immunotherapu drugs fail- Cancer Research UK
Tumor-dwelling immune cells thwart cancer immunotherapy- Medical News Today
Molecular imaging reveals mechanism for resistance to immune checkpoint blockade- eCancer News
Cell Surface Cleaning to Boost Effect of Medications – Technology.org
Secret reasons for immunotherapy failure: antibodies taken up by macrophages – Sohu (in chinese)
Descubrieron mecanismo de resistencia inmunitaria del cáncer – segundoenfoque (in spanish)
PD-1 blockade: It’s what’s for dinner – Science Immunology
Macrophages hijack anti-PD-1 therapy – Nature Reviews Clinical Oncology
Macrophages Promote Resistance to Checkpoint Inhibitors – Cancer Discovery
Macrophages steal the show – Nature Reviews Cancer