Pancreatic Cancer Wars: A New Hope
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest types of cancers – with only a 7% survival rate after 5 years. A major factor behind this staggering statistic is the often late detection of disease due to a lack of reliable early tests specific for pancreatic cancer. Researchers from the MGH Center for Systems Biology along with MGH Cancer Center colleagues have now developed a simple, robust, and rapid blood-based test to diagnose PDAC. Access to the article and press coverage is included below.
Extracellular vesicles (EVs) are tiny (50-100 nm) particles shed from all cell types into the circulation. These vesicles contain genetic information highly similar to the cell from which they originated. Importantly, they can be purified from a routine blood draw. Tumor cells are known to shed significantly more EVs, providing a potential wealth of information about the tumor without the need for an invasive test. However, a significant challenge in using EVs for diagnoses is the lack of sensitive assays for analyzing molecular contents of EVs.
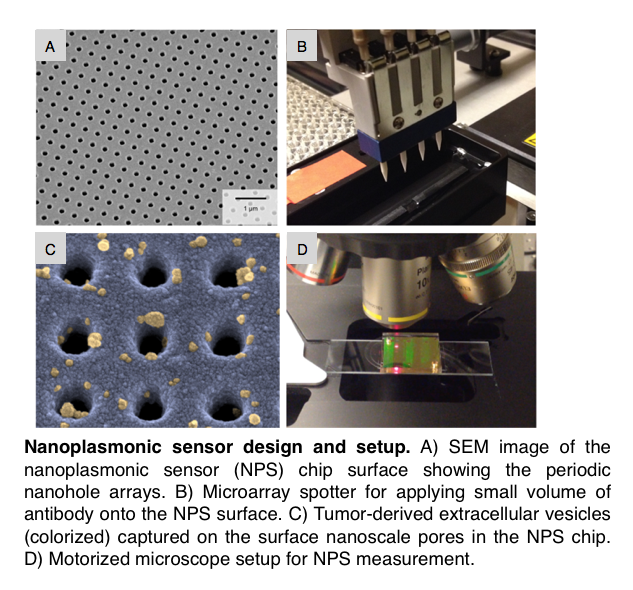
Dr. Hyungsoon Im, a co-first author on the study, leveraged his extensive biomedical engineering expertise, to create a nanoplasmonic sensor, dubbed “NPS”, that can measure dozens of protein biomarkers in EVs with high sensitivity and throughput. The NPS sensor comprises a silicon chip coated with a thin layer of gold and thousands of nanopores that exhibit unique light transmission through the pores. Antibodies against pancreatic cancer biomarkers are immobilized on the nanopores. EVs containing the protein biomarkers of interest are then captured at these nanopores. Light transmitted through the pores shifts in the presence of tumor-derived EVs, a change that Dr. Im and his team can easily measure.
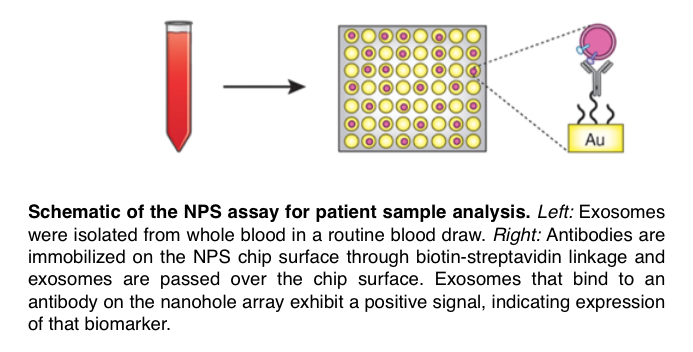
To capitalize on NPS technology for pancreatic cancer detection, the team analyzed EVs purified from patient blood for multiple cancer markers to correctly identify pancreatic cancer. Preliminary testing led to identification of a five protein marker signature (“PDACEV”) that correctly identified pancreatic cancer. In a larger group of patient samples, the PDACEV signature showed 84% accuracy. This novel signature is now poised to be tested in larger patient populations, particularly high-risk patients with a family history of disease, to explore its potential in the PDAC screening space.
Article
Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, Yang R, Castro CM, Lee H, Del Castillo CF, Weissleder R
Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy
Sci Transl Med. 2017;9(391):eaaI3226
Press coverage
Blood Test for Earlier Detection of Pancreatic Cancer Feasible – Physician’s Briefing
Blood test for pancreatic cancer shows early promise – Health Medicine Network
New test may improve pancreatic cancer diagnoses – ScienceNews
Blood Test for Pancreatic Cancer Shows Early Promise – HealthDay
Harvard-MGH Liquid Biopsy Assay Targets Early Detection of Pancreatic Cancer – 360Dx