Macrophage-targeted nanoparticles improve cancer immunotherapy
Tumor associated macrophages (TAMs) are highly abundant in a broad range of cancers, often promoting tumor growth, metastasis, and therapeutic resistance as a result of their pro-tumorigenic M2-like phenotype. This recently developed understanding has motivated great interest in macrophage-directed immunotherapies, including such methods as altered recruitment, depletion, and re-education. Of these approaches, only re-education to a M1-like phenotype provides the ability to promote anti-tumor immune response. However, challenges remain in the identification of therapeutic drugs which drive TAM re-education with high potency, as well as in the ability to preferentially deliver these therapeutics to TAMs.
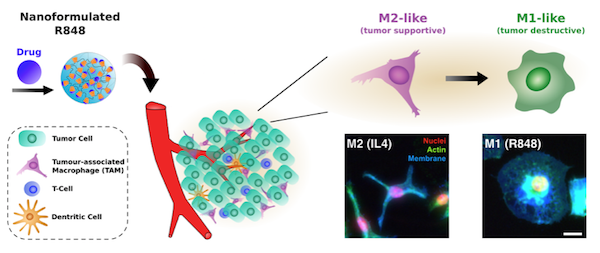
In order to identify potent therapeutics for TAM re-education, researchers at the CSB developed a high-content image based screen of macrophage phenotype. These techniques enabled quantitative comparison of macrophage polarizing drugs, thereby identifying an endosomal toll-like receptor agonist (R848) as a highly potent driver of M1-like re-education in vitro.
To assist in TAM-targeted therapy, the researchers furthermore developed a cyclodextrin nanoparticle as a supramolecular drug delivery vehicle, aiding in drug solublization and delivery to macrophages. The drug-loaded nanoparticle demonstrated an ability to re-educate both murine and human macrophages in vitro with greater potency than the free drug alone.
To investigate the behavior of the drug-loaded nanoparticle in vivo, fluorescent derivatives of both the drug and nanoparticle were used in conjunction with real-time intravital microscopy. Rapid uptake of the nanoparticle by TAMs in vivo enhanced local drug delivery, resulting in robust acquisition of an anti-tumor TAM phenotype. This observation is of vital importance, as clinical use of toll-like receptor agonists has typically been limited to topical application due to the poor bioavailability of the small molecule drugs at the target site within the tumor.
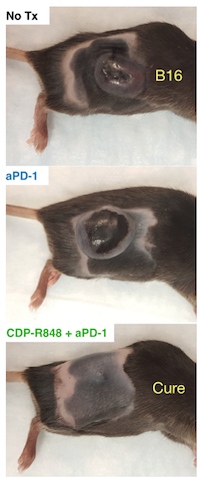
Finally, the team investigated the efficacy of the TAM re-education therapy in vivo. As a monotherapy, the nanoformulation controlled tumor growth in mice. Combination therapy with anti-PD-1 checkpoint therapy improved immunotherapy response rates, remarkably improving treatment efficacy. These findings demonstrate the potential for effective modulation of the innate immune system (i.e., macrophages) to act in cooperation with standard of care checkpoint immunotherapies, boosting anti-cancer response. In sum, these studies demonstrate the potential of TAM re-education as a viable therapeutic strategy in cancer treatment, while highlighting the need for nanotherapeutic platforms with high TAM affinity.
Article
Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R
TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy
Nature Biomedical Engineering. 2018; DOI: 10.1038/s41551-018-0236-8
Press Coverage
Behind the paper – Re-educating tumor macrophages for immunotherapy – Nature Biomedical Engineering Community
Immune Cell Biology newsletter feature – Accelerating Cancer Immunotherapy Research (ACIR)
Sugar Nanoparticles Reprogram Immune Cells to Help Destroy Tumors – Medgadget
Cancer: Re-educating tumour-associated macrophages with nanoparticles – Nature Reviews Drug Discovery