Fluorescence Molecular Tomography (FMT) imaging facility
Two FMT systems are available at the CSB for imaging mice in vivo using a variety of near-infrared fluorescent imaging agents. The systems are equipped with isoflurane anesthesia and are heated to maintain subject body temperature during imaging.
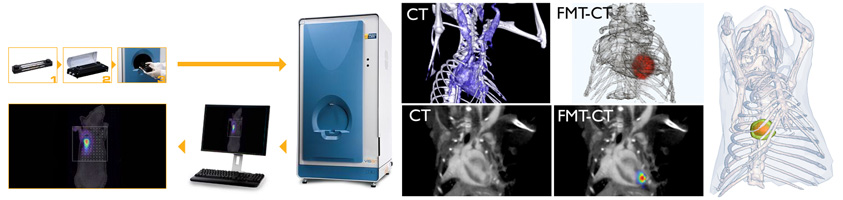
There are four fluorescent channels available for imaging (635/655 nm, 680/700 nm, 750/780 nm, 785/815nm). The FMT software allows for quantification of fluorescence in a three-dimensional region of interest. While the systems are equipped to capture planar images both in white light and fluorescence, they are also able to detect fluorescence deep within tissue and to resolve probe signal in organs such as the lungs and the heart.
Typically, we combine FMT imaging, which yields a three-dimensional map of fluorochrome tracer concentration, with anatomical imaging (CT or MRI). This approach, akin to PET-CT imaging, can simultaneously deliver molecular information (FMT) as well as the exact anatomic structure from which the signal originates (CT, as shown in figures).
Imaging is rapid. A typical FMT acquisition takes approximately 5 minutes and reconstructions of the data sets are run on the system. Region of interest data can be collected and exported to an Excel spreadsheet, and screenshots and Z-stack movies can also be created.
Fluorescence Protein Tomography (FPT) imaging
FPT is a variation of FMT that is optimized for the in vivo three-dimensional tomographic imaging of fluorescent proteins in mice. This method essentially extends everyday fluorescent protein tagging techniques into the realms of three-dimensional non-invasive in vivo small animal imaging.
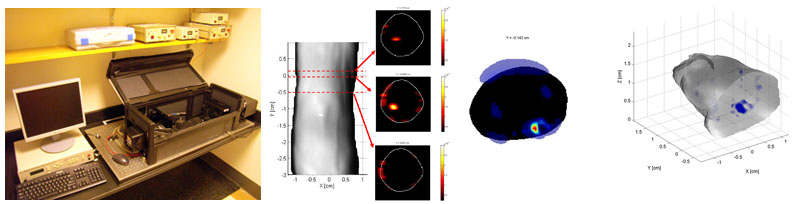
The third generation of the FPT system is highly versatile. Tomographic imaging can be performed (a) with or without the use of index matching fluid, (b) by incorporating either a single planar projection or multiple full-angular projections of the mouse with surface extraction, and © with a sequence of filters that obtain multispectral acquisitions. The system is equipped with 3 lasers (tunable Ar+, 532nm NdYAG, 593nm DPSS) to excite a wide variety of fluorescent proteins (from green fluorescent proteins to the new red shifted fluorescent proteins), and is also equipped with the standard 670 and 750 nm diode lasers for excitement of near-infrared fluorescent probes.
Mesoscopic Fluorescence Tomography (MFT)
Optical imaging methods are currently inadequate for non-invasive in vivo imaging of intact developing insects, animal embryos or small animal extremities, i.e. when working at a scale that lies between the penetration limits of modern optical microscopy (0.5-1mm) and the diffusion-imposed limits of optical macroscopy (>1cm).
Mesoscopic Fluorescence Tomography (MFT) was developed to operate at the 0.5mm-1cm scale to enable in vivo observation of common biological model organisms. The technique utilizes a modified laboratory microscope and multi-projection illumination to collect data at 360-degree projections. It employs the Fermi simplification to the Fokker-Plank solution of the photon transport equation. This is then combined with geometrical optic principles in order to allow in vivo whole-body visualization of non-transparent three-dimensional structures in samples of up to several millimeters in size.
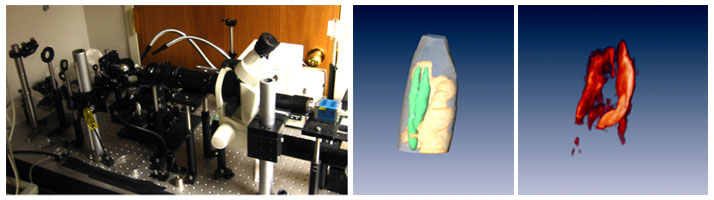
To investigate the in vivo performance of the technique, we have applied it to the imaging of developing Drosophila. The Drosophila are an extensively studied species that have gained high importance in biomedical research. Yet since most studies to date rely on histological sectioning, it has not been possible to study the evolution of events in response to mutations and external stimuli in a high-throughput fashion. In addition, this method requires a large number of insects in order to accurately build up a time sequence of events. Using MFT, however, we have now been able to show that it is possible to three-dimensionally visualize GFP-expressing salivary glands. Furthermore, we have also studied the morphogenesis of wing-imaginal discs in vivo and in real time over a period of six consecutive hours.
This new-found ability offers the dimension of “time” in the study of developing insects. It also increases our ability to interrogate events in an unperturbed environment and longitudinally in the same insect.