Breton Lab
Read BioOur laboratory studies the regulation of epithelial cells in various organs of the urogenital tract, with an emphasis on the kidney and epididymis. We developed a multidisciplinary approach including high-resolution laser scanning confocal microscopy, and intravital multiphoton microscopy, 3D reconstruction of single cells, luminal perfusion of the epididymis in vivo, and monitoring of live cells. We study the function of epithelial cells while they reside in their native environment. By comparing and contrasting the kidney and epididymis epithelia, we revealed novel signaling pathways that contributed to our better understanding of how specialized proton-secreting cells, the collecting duct intercalated cells and epididymal clear cells, establish and maintain an acidic luminal environment, which is essential for whole body acid/base homeostasis by the kidney, and sperm maturation and storage in the epididymis. In addition, our recent study showed novel functions for intercalated cells as sensors and mediators of sterile inflammation. We also revealed a previously unrecognized role for CFTR, whose mutations cause cystic fibrosis, as a crucial regulator of epithelial tubulogenesis and remodeling.
Recent Publications
Mendelsohn AC, Sanmarco LM, Spallanzani RG, Brown D, Quintana FJ, Breton S, Battistone MA
From initial segment to cauda: A regional characterization of mouse epididymal CD11c+ mononuclear phagocytes based on immune phenotype and function. 2020;319(6):C997-C1010 - PMID: 32991210 - PMCID: PMC7792679 - DOI: 10.1152/ajpcell.00392.2020Castro MM, Kim B, Games PD, Hill E, Neves CA, Serrão JE, Breton S, Machado-Neves M
Distribution pattern of ZO-1 and claudins in the epididymis of vampire bats. Tissue Barriers. 2020;8(3):1779526 - PMID: 32552339 - PMCID: PMC7549744 - DOI: 10.1080/21688370.2020.1779526Battistone MA, Mendelsohn AC, Spallanzani RG, Allegretti AS, Liberman RN, Sesma J, Kalim S, Wall SM, Bonventre JV, Lazarowski ER, Brown D, Breton S
Pro-inflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice. J Clin Invest. 2020;130(7):3734-3749 - PMID: 32287042 - PMCID: PMC7324186 - DOI: 10.1172/JCI134791Hoyer FF*, Naxerova K*, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, Wojtkiewicz G, Rohde D, Frodermann V, Vandoorne K, Courties G, Iwamoto Y, Garris CS, Williams DL, Breton S, Brown D, Whalen M, Libby P, Pittet MJ, King KR, Weissleder R, Swirski FK, Nahrendorf M
Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity. 2019;51(5):899-914.e7 - PMID: 31732166 - PMCID: PMC6892583 - DOI: 10.1016/j.immuni.2019.10.010Battistone MA, Spallanzani RG, Mendelsohn AC, Capen D, Nair AV, Brown D, Breton S
Novel role of proton-secreting epithelial cells in sperm maturation and mucosal immunity. J Cell Sci. 2019;133(5):ePub - PMID: 31636115 - PMCID: PMC7003979 - DOI: 10.1242/jcs.233239- More publications ...
Research projects
Luminal acidification and its regulation
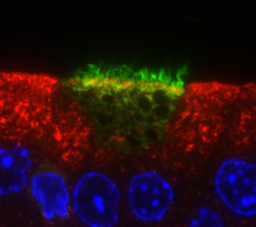 The kidney and the epididymis are tubular organs that both must regulate transepithelial transport and luminal pH. Therefore, they contain comparable epithelial cells that perform similar functions. Many of the cell-cell communication networks and intracellular processes, which we first discovered in the epididymis, are recapitulated in the kidney. We showed that proton secretion in a sub-population of epididymal epithelial cells, the clear cells, is regulated via recycling of the vacuolar H+ATPase (V-ATPase), a process that is modulated by luminal pH, bicarbonate, and circulating aldosterone. Recycling of V-ATPase to and from the apical membrane also occurs in type A intercalated cells of the kidney, where systemic acid/base balance is maintained by pumping protons into the urine to remove acid from the body. We have identified a novel pathway for the sensing and regulation of extracellular pH via a physiological link between the bicarbonate activated soluble adenylyl cyclase (sAC) and the V-ATPase. We showed that PKA is a down-stream effector of sAC-dependent elevation of cAMP in the epididymis and the kidney. We are currently examining the purinergic regulation of epididymal clear cells and renal intercalated cells. The clinical relevance of this discovery research project is related to the fact that, in the United States, millions of couples are infertile. We believe that a lack of knowledge regarding epididymal biology contributes to the fact that many cases of male infertility still remain unexplained. A major cause of male infertility is the production of sperm that have reduced function, including low motility and poor interaction with oocytes. Acidic pH and low bicarbonate contribute to the maturation and storage of spermatozoa in the epididymis. Thus, a defect in the acidification capacity of the epididymis has important consequences for male reproductive physiology. In addition, defective V-ATPase function in renal intercalated cells lead to distal renal tubular acidosis and the formation of kidney stones.
The kidney and the epididymis are tubular organs that both must regulate transepithelial transport and luminal pH. Therefore, they contain comparable epithelial cells that perform similar functions. Many of the cell-cell communication networks and intracellular processes, which we first discovered in the epididymis, are recapitulated in the kidney. We showed that proton secretion in a sub-population of epididymal epithelial cells, the clear cells, is regulated via recycling of the vacuolar H+ATPase (V-ATPase), a process that is modulated by luminal pH, bicarbonate, and circulating aldosterone. Recycling of V-ATPase to and from the apical membrane also occurs in type A intercalated cells of the kidney, where systemic acid/base balance is maintained by pumping protons into the urine to remove acid from the body. We have identified a novel pathway for the sensing and regulation of extracellular pH via a physiological link between the bicarbonate activated soluble adenylyl cyclase (sAC) and the V-ATPase. We showed that PKA is a down-stream effector of sAC-dependent elevation of cAMP in the epididymis and the kidney. We are currently examining the purinergic regulation of epididymal clear cells and renal intercalated cells. The clinical relevance of this discovery research project is related to the fact that, in the United States, millions of couples are infertile. We believe that a lack of knowledge regarding epididymal biology contributes to the fact that many cases of male infertility still remain unexplained. A major cause of male infertility is the production of sperm that have reduced function, including low motility and poor interaction with oocytes. Acidic pH and low bicarbonate contribute to the maturation and storage of spermatozoa in the epididymis. Thus, a defect in the acidification capacity of the epididymis has important consequences for male reproductive physiology. In addition, defective V-ATPase function in renal intercalated cells lead to distal renal tubular acidosis and the formation of kidney stones.
Renal inflammation and acute kidney injury (AKI)
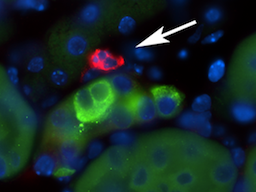 Uncontrolled inflammation is one of the leading causes of acute kidney injury (AKI). Regardless of the cause of hospitalization, all patients admitted to the intensive care unit (ICU) are at a high risk of developing AKI. On a yearly basis, up to two thirds of the 5.7 millions of ICU patients in the USA develop AKI. Serum creatinine, the most utilized AKI marker, becomes elevated only after most of the kidney function is lost. Consequently, the onset of AKI often remains undetected. This underscores the need for a biomarker that can detect kidney injury early, and treatment that can be administered while damage can still be prevented. How diverse organs communicate damage to the kidney, leading to AKI, is of great current interest. We showed that in addition to their central role in the regulation and maintenance of acid-base balance via the proton-pumping activity of the vacuolar H+-ATPase (V-ATPase), renal type A intercalated cells (A-ICs) are also sensors that mediate sterile inflammation in the kidney medulla. This resulted from our identification of high expression levels of the pro-inflammatory UDP-glucose receptor P2Y14 in A-ICs. We showed that UDP-glucose is a “danger-signal” molecule that triggers expression of pro-inflammatory chemokines in A-ICs, which attract neutrophils into the renal medulla. We are currently investigating the role of UDP-glucose as a biological mediator that initiates inter- and intra-organ crosstalk, which ultimately induces renal inflammation.
Uncontrolled inflammation is one of the leading causes of acute kidney injury (AKI). Regardless of the cause of hospitalization, all patients admitted to the intensive care unit (ICU) are at a high risk of developing AKI. On a yearly basis, up to two thirds of the 5.7 millions of ICU patients in the USA develop AKI. Serum creatinine, the most utilized AKI marker, becomes elevated only after most of the kidney function is lost. Consequently, the onset of AKI often remains undetected. This underscores the need for a biomarker that can detect kidney injury early, and treatment that can be administered while damage can still be prevented. How diverse organs communicate damage to the kidney, leading to AKI, is of great current interest. We showed that in addition to their central role in the regulation and maintenance of acid-base balance via the proton-pumping activity of the vacuolar H+-ATPase (V-ATPase), renal type A intercalated cells (A-ICs) are also sensors that mediate sterile inflammation in the kidney medulla. This resulted from our identification of high expression levels of the pro-inflammatory UDP-glucose receptor P2Y14 in A-ICs. We showed that UDP-glucose is a “danger-signal” molecule that triggers expression of pro-inflammatory chemokines in A-ICs, which attract neutrophils into the renal medulla. We are currently investigating the role of UDP-glucose as a biological mediator that initiates inter- and intra-organ crosstalk, which ultimately induces renal inflammation.
Chronic kidney disease (CKD) associated with cystic fibrosis
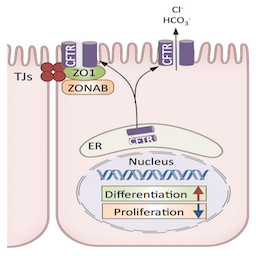 As cystic fibrosis (CF) patients receive better treatments that improve their life expectancy, they have now begun to develop progressive malfunction of their kidneys. Consequently, the prevalence of CKD associated with CF is now twice that of the general population. Using the epididymis as a model system, we revealed a previously unrecognized role for CFTR, the protein whose mutations cause CF, as a crucial regulator of epithelial tubulogenesis and remodeling. We showed that CFTR regulates the proliferation/differentiation switch during tubulogenesis. Through its interaction with the tight junction (TJ) protein ZO1, CFTR contributes to the retention of the transcription factor ZONAB outside the nucleus, in TJs, leading to decrease in proliferation and activation of differentiation. Our recent findings, thus, provide a new paradigm for the etiology of diseases associated with CFTR mutations, including cystic fibrosis. We are currently investigating the possibility that disruption of the CFTR/ZO1/ZONAB interaction leads to a reduced capacity of the kidney to re-differentiate after injury.
As cystic fibrosis (CF) patients receive better treatments that improve their life expectancy, they have now begun to develop progressive malfunction of their kidneys. Consequently, the prevalence of CKD associated with CF is now twice that of the general population. Using the epididymis as a model system, we revealed a previously unrecognized role for CFTR, the protein whose mutations cause CF, as a crucial regulator of epithelial tubulogenesis and remodeling. We showed that CFTR regulates the proliferation/differentiation switch during tubulogenesis. Through its interaction with the tight junction (TJ) protein ZO1, CFTR contributes to the retention of the transcription factor ZONAB outside the nucleus, in TJs, leading to decrease in proliferation and activation of differentiation. Our recent findings, thus, provide a new paradigm for the etiology of diseases associated with CFTR mutations, including cystic fibrosis. We are currently investigating the possibility that disruption of the CFTR/ZO1/ZONAB interaction leads to a reduced capacity of the kidney to re-differentiate after injury.
Three-dimensional modeling of basal cell function in pseudostratified epithelia
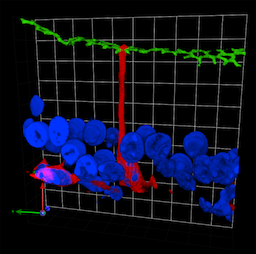 Many organs in the body, including those of the reproductive tract and the lungs, are comprised of a system of tubules lined by epithelial cells. The prevailing view is that so-called “basal cells” in these epithelia are never in contact with the fluid or air-filled cavity (known as the lumen), but we showed that these cells in fact extend long, slender projections, which we named “axiopodia”, that scan the luminal content. We showed that basal cells modulate organ function by communicating their findings to adjacent cells via the local production of nitric oxide. Using multi-photon in vivo microscopy, we made the surprising discovery that basal cell axiopodia periodically extend and retract over time. We found that axiopodia extensions and retractions follow an oscillatory pattern. This movement, which we referred to as periodic axial motility (PAM), is controlled by c-Src and MEK1/2-ERK1/2. Therapeutic inhibition of tyrosine kinase activity induces a retraction of these projections. Such unexpected cell motility may reflect a novel mechanism by which specialized epithelial cells sample the luminal environment. We have also found basal cell axiopodia in several epithelia, including the trachea, olfactory mucosa, prostate and seminal vesicles, showing that the luminal sampling property of basal cells might be a generalized phenomenon. Our research will promote new diagnostic and therapeutic strategies for diseases including male infertility, chronic obstructive airway disease, asthma and cystic fibrosis.
Many organs in the body, including those of the reproductive tract and the lungs, are comprised of a system of tubules lined by epithelial cells. The prevailing view is that so-called “basal cells” in these epithelia are never in contact with the fluid or air-filled cavity (known as the lumen), but we showed that these cells in fact extend long, slender projections, which we named “axiopodia”, that scan the luminal content. We showed that basal cells modulate organ function by communicating their findings to adjacent cells via the local production of nitric oxide. Using multi-photon in vivo microscopy, we made the surprising discovery that basal cell axiopodia periodically extend and retract over time. We found that axiopodia extensions and retractions follow an oscillatory pattern. This movement, which we referred to as periodic axial motility (PAM), is controlled by c-Src and MEK1/2-ERK1/2. Therapeutic inhibition of tyrosine kinase activity induces a retraction of these projections. Such unexpected cell motility may reflect a novel mechanism by which specialized epithelial cells sample the luminal environment. We have also found basal cell axiopodia in several epithelia, including the trachea, olfactory mucosa, prostate and seminal vesicles, showing that the luminal sampling property of basal cells might be a generalized phenomenon. Our research will promote new diagnostic and therapeutic strategies for diseases including male infertility, chronic obstructive airway disease, asthma and cystic fibrosis.
News
Sylvie Breton has been named the inaugural incumbent of the Richard Moerschner Endowed MGH Research Institute Chair in Men’s Health. Congratulations, Sylvie!
CSB PMB work on high resolution imaging of the kidney using helium ion microscopy (Rice et al., PLoSONE 8(3); e57051, 2013) was featured recently in the annual report from the NIDDK (Recent Advances and Emerging Opportunities) under the heading “A closer look at kidney structure”. (pdf)
CSB is delighted to announce that Sylvie Breton, PhD has been promoted to Professor of Medicine, Harvard Medical School. This makes her one of the rare PhD professors in this department.
Sylvie Breton, Ph. D., is the 2012 recipient of the highly prestigious "Research Award" from the Society for the Study of Reproduction. This annual award recognizes research excellence in the field of reproduction, based on innovation and originality in addition to rewarding leadership and participation in other scholarly activities. Congratulations Sylvie!
Sylvie Breton, PhD has been named the 2011 MGH Research Scholars for work on intercellular communication through novel cell structures.