PAST RESEARCH PROJECTS
The microbiome and pathogen diagnostics
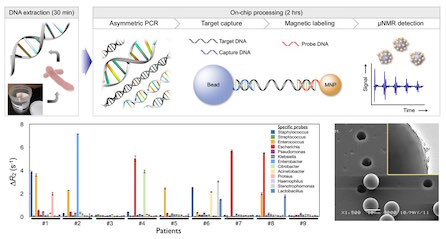
Our gut and body is colonized by hundreds of microbial species that have important immune modulating functions in health and disease. The composition of the intestinal micro biome has been associated with autoimmune disorders such as rheumatoid arthritis and diabetes, obesity, transplantation, resistance to systemic cancer therapy and the efficacy of immunotherapies. Many antibiotics destroy intestinal microbial communities and increase the susceptibility to deadly pathogens. Furthermore the incriminate use of antibiotics and escalating resistance increases morbidity, mortality, and healthcare costs. While sequencing of bacterial genomes and 16sRNA has enabled modern microbiome research, it is less practical in routine clinical settings where diagnostic results are required in hours. We thus need advanced diagnostics to enable rapid, sensitive, specific, culture-independent species identification of key hospital-associated pathogens. Such advanced diagnostics would i) allow clinicians to quickly determine the most effective treatment for infected individuals, ii) support antimicrobial stewardship by reducing the empiric use of broad-spectrum antimicrobials, and iii) facilitate enrolling target populations in pivotal efficacy trials of new antibiotics, thereby reducing the size and cost of antibacterial clinical trials. We have developed advanced bacterial profiling technologies capable of single bacterial detection sensitivities, genomic phenotyping and measurements of drug resistance genes. We continue to apply them to important clinical and biological questions. We anticipate this new diagnostic technology will enable rapid, culture-independent detection of high-priority antimicrobial-resistant Gram-negative bacterial pathogens and thus address the epidemic of healthcare-associated infections. Some of our technologies have also been commercialized and are FDA approved (T2Biosystems:http://www.t2biosystems.com).
- Chung HJ, Castro CM, Im H, Lee H, Weissleder R. A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nature Nanotechnol. 2013;8(5):369-75 – PMID: 23644570 – PMCID: PMC3711657
- Liong M, Hoang AN, Chung J, Gural N, Ford CB, Min C, Shah RR, Ahmad R, Fernandez-Suarez M, Fortune SM, Toner M, Lee H*, Weissleder R* Magnetic barcode assay for genetic detection of pathogens. Nat Commun. 2013;4:1752 – PMID: 23612293 – PMCID: PMC3635151
Nanomaterials
Nanoscale materials and devices hold great promise for advanced diagnostics, sensors, targeted drug delivery, smart drugs, screening and novel cellular therapies. The Nanotechnology Program at CSB develops, optimizes and validates creative approaches to diagnosis and treatment of human disorders. The Nanotechnology Program at CSB is funded through two NIH designated Centers of Excellence:
Both programs are multidisciplinary and interinstitutional involving investigators from MIT, Harvard University, Harvard Medical School, Harvard affiliated teaching hospitals and the Broad Institute.