Pai Lab
Read BioOur research mission is to rapidly translate innovative, biomarker driven immunotherapies that have the potential to improve treatment outcomes for patients with head and neck cancer. We have a particular interest in studying human papillomavirus (HPV) induced tumors as a model system both for understanding immune evasion mechanisms utilized by cancer cells and for tracking the modulation of tumor- (or HPV-) specific immune responses with various applied therapies.
Our translational research laboratory has two broad areas of focus: the development of novel immunomodulatory therapies for head and neck cancer patients and the discovery and clinical application of diagnostic and/or predictive biomarkers.
Recent Publications
Qin T, Mattox AK, Campbell JS, Park JC, Shin KY, Li S, Sadow PM, Faquin WC, Micevic G, Daniels AJ, Haddad R, Garris CS, Pittet MJ, Mempel TR, ONeill A, Sartor MA, Pai SI
Epigenetic therapy sensitizes anti-PD-1 refractory head and neck cancers to immunotherapy rechallenge. J Clin Invest. 2025;135(6):ePub - PMID: 40091844 - PMCID: PMC11910227 - DOI: 10.1172/JCI181671Bill R, Wirapati P, Messemaker M, Roh W, Zitti B, Duval F, Kiss M, Park JC, Saal TM, Hoelzl J, Tarussio D, Benedetti F, Tissot S, Kandalaft L, Varrone M, Ciriello G, McKee TA, Monnier Y, Mermod M, Blaum EM, Gushterova I, Gonye ALK, Hacohen N, Getz G, Mempel TR, Klein AM, Weissleder R, Faquin WC, Sadow PM, Lin D, Pai SI, Sade-Feldman M, Pittet MJ
CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science. 2023;381(6657):515-524 - PMID: 37535729 - PMCID: PMC10755760 - DOI: 10.1126/science.ade2292Clifton GT, Rothenberg M, Ascierto PA, Begley G, Cecchini M, Eder JP, Ghiringhelli F, Italiano A, Kochetkova M, Li R, Mechta-Grigoriou F, Pai SI, Provenzano P, Puré E, Ribas A, Schalper KA, Fridman WH
Developing a definition of immune exclusion in cancer: results of a modified Delphi workshop. J Immunother Cancer. 2023;11(6):ePub - PMID: 37290925 - PMCID: PMC10254706 - DOI: 10.1136/jitc-2023-006773Pai SI, Matheus HR, Guastaldi FPS
Effects of periodontitis on cancer outcomes in the era of immunotherapy. Lancet Healthy Longev. 2023;4(4):e166-e175 - PMID: 37003275 - PMCID: PMC10148268 - DOI: 10.1016/S2666-7568(23)00021-1Sun Y, Revach OY, Anderson S, Kessler EA, Wolfe CH, Jenney A, Mills CE, Robitschek EJ, Davis TGR, Kim S, Fu A, Ma X, Gwee J, Tiwari P, Du PP, Sindurakar P, Tian J, Mehta A, Schneider AM, Yizhak K, Sade-Feldman M, LaSalle T, Sharova T, Xie H, Liu S, Michaud WA, Saad-Beretta R, Yates KB, Iracheta-Vellve A, Spetz JKE, Qin X, Sarosiek KA, Zhang G, Kim JW, Su MY, Cicerchia AM, Rasmussen MQ, Klempner SJ, Juric D, Pai SI, Miller DM, Giobbie-Hurder A, Chen JH, Pelka K, Frederick DT, Stinson S, Ivanova E, Aref AR, Paweletz CP, Barbie DA, Sen DR, Fisher DE, Corcoran RB, Hacohen N, Sorger PK, Flaherty KT, Boland GM, Manguso RT, Jenkins RW
Targeting TBK1 to overcome resistance to cancer immunotherapy. Nature. 2023;615(7950):158-167 - PMID: 36634707 - PMCID: PMC10171827 - DOI: 10.1038/s41586-023-05704-6- More publications ...
Research projects
Development of novel immunomodulatory therapies for head and neck cancer patients
The approach that we have taken to this area of translational research is to develop various novel immunodulatory therapies which targets the spectrum of head and neck cancer care, which includes the recurrent and/or metastatic, newly diagnosed, and the pre-malignant setting. Some active areas of research for these different stages of cancer include:
Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma Patients
Our laboratory seeks to investigate the tumor immune microenvironment of HPV-associated head and neck cancers in the context of PD-1:PD-L1 pathway activation with a goal of identifying additional targetable pathways for clinical translation. We validate the identified targets in a larger cohort of patient samples as well as assess the impact of targeted blockade alone and in combination with anti-PD1 in pre-clinical cancer models which assists in informing the appropriate targets for translation into head and neck cancer patients.
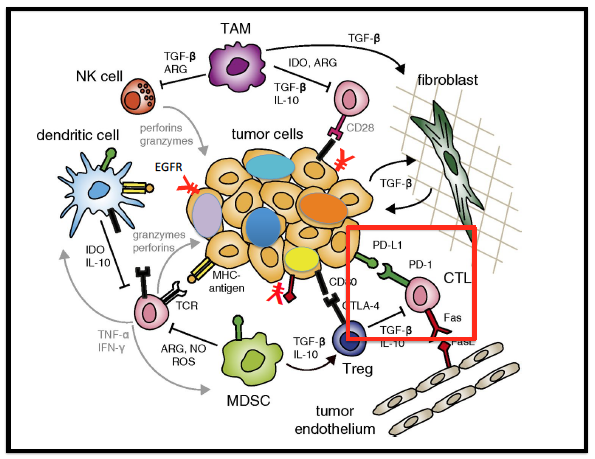
Our translational research interest to identify and overcome mechanisms of immune resistance in head and neck cancer is the major focus of a program project (P01) grant. The goal of our P01 grant is to build upon the incremental successes of immunotherapy and improve clinical outcomes by addressing two main barriers to achieving clinical response to immunotherapy: (a) the tumor’s overall poor antigenicity (intrinsic mechanism), which limits the generation of antitumor immunity, and (b) innate and adaptive immune suppression (extrinsic mechanism) that results in immune tolerance. One specific projectfocuses on reprogramming the antigenicity of head and neck cancers through epigenetic therapy with the goal of enhancing overall tumor antigen presentation and recognition by the immune system. We hypothesize that a DNA methyltransferase inhibitor can uncover epigenetically silenced genes, such as HLA class I antigen processing machinery (APM) components and neo-antigens across and within heterogeneous tumor cell populations, to uniformly improve tumor cell antigenicity and, thus, immunogenicity which can translate into clinical benefit. This hypothesis is being evaluated in an ongoing phase 1 clinical trial (NCT 03019003).
In addition, our group is assessing whether a novel immunomodulatory fusion protein, CUE-101, can increase the frequency and function of tumor infiltrating HPV-16 specific T cells. The backbone of CUE-101 is the Immuno-STAT (Selective Targeting and Alteration of T cells) platform that utilizes a modular framework that consists of an interchangeable HLA complex with an interchangeable bound tumor-associated antigen (TAA) peptide epitope. In addition, through enzymatic linkers, the fusion protein can locally deliver any cytokine(s), ligand(s), and/or co-stimulatory molecule to directly stimulate and activate tumor-specific T cells in vivo. Therefore, we are investigating directly in human subjects whether the CUE-101 platform, either alone or in combination with pembrolizumab, is able to reinvigorate pre-existing systemic and/or local HPV-(or tumor)-specific immune responses as well as induce memory HPV-specific T cells which can translate into prolonged disease free survival (DFS) (NCT 03978689).
Pre-Malignant HPV-associated Recurrent Respiratory Papillomas
We have an ongoing therapeutic clinical trial administering immunotherapy to patients with pre-malignant tumors. Recurrent Respiratory Papillomatosis (RPP) is caused by infection with human papillomavirus (HPV) types 6 and 11 and is characterized by mucosal tumors that grow in the respiratory tract, impacting breathing, swallowing, and speech. In 3-5% of patients, the lesions can undergo malignant transformation and the prognosis for patients who develop cancers derived from their RRP is poor due to the limited treatment options available. There is no effective systemic therapy for RRP, despite the fact that it typically involves multiple anatomic subsites. Thus, there is a strong unmet clinical need to develop novel treatment options for RRP patients. We and others have shown that RPP is a disease characterized by an ineffective host immune response to HPV, in part, through the upregulation of the PD1:PDL1 axis. Thus, we hypothesized that administration of a blocking PD-1 monoclonal antibody, pembrolizumab, can re-activate host anti-tumor responses, leading to the regression of existing RPP lesions, clearance of chronic HPV infection, and prevention of new disease as a means to improve the quality of life and achieve possible cure. To test this hypothesis, we opened an investigator-initiated phase II clinical trial administering pembrolizumab to subjects diagnosed with advanced RRP (DFHCC IRB#15-469; NCT02632344) to evaluate the effectiveness of immunotherapy as a novel treatment option. We have completed up to 2-years of treatment of all patients in January 2021 and we are currently analyzing the serial peripheral blood and tumor biopsy samples obtained from each treated patient. Utilizing RNASequencing, proteomic microarrays, HPV tetramer staining followed by flow cytometry, and multiplex immunofluorsecence staining, we are assessing HPV-specific T cell modulation with long term treatment with immunotherapy.
The discovery and clinical application of diagnostic and/or predictive biomarkers
Given the unprecedented growth in biotechnology and pharmaceutical drug development in the past 10 years surrounding IO drugs, both clinicians and patients are overwhelmed with the multitude of currently available treatment options. Thus, there are increasing efforts to discover and integrate predictive and/or prognostic biomarkers into treatment algorithms in order to optimize cancer care (Pai SI et al J Immunother Cancer 2020). Over the next 5-10 years, this is the area of greatest potential growth and clinical impact in the IO field which is the rationale for it being the second major focus of our translational research efforts. Utilizing scRNAseq, genomics, mouse models and human tissue, we have ongoing collaborative efforts to identify and prospectively validate new diagnostic and predictive biomarkers of response to immunotherapy in a variety of cancers.
News
Sara Pai, MD, PhD, director of the Translational Research in Head and Neck Cancer, have been elected to the American Society for Clinical Investigation.