Research Focus
Imagine, if we understood what the 30 trillions of cells in our body do at any given moment...
Now compare these 30 trillions of cells (not counting the microbiome!) to the earth population of 8B people (3,750 times more!). This creates a massive undertaking...
At CSB we develop innovative technologies to enable the discovery of new biology, drug targets and diagnostics.


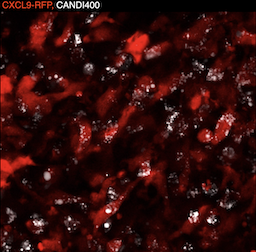
Coaxing macrophages to fight cancer
Our previous work found that high levels of tumor macrophages expressing the chemokine CXCL9 are associated with good clinical outcomes in cancer patients. Unfortunately, most tumor-associated macrophages do not express CXCL9. In this study, we develop a macrophage image screening approach to identify drugs that potentiate CXCL9 production. Here, we show a triplet drug combination incorporated into a macrophage-avid nanoparticle that strongly enhances macrophage CXCL9 production and triggers remarkable anti-tumor efficacy in mouse cancer models. We envision leveraging deep molecular analysis of anti-tumor macrophages with novel drug screening methods to develop new cancer treatment methods. Learn more...
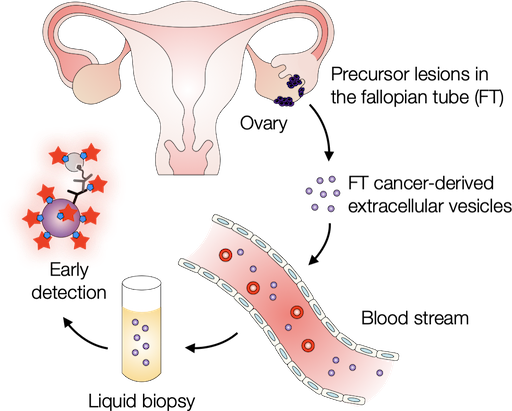
Probing extracellular vesicles promises early detection of ovarian cancer
Ovarian cancer poses challenges for early detection due to its nonspecific symptoms, often resulting in late-stage diagnosis. This study aimed to address this issue by investigating extracellular vesicles (EVs) as an early biomarker for ovarian cancer, specifically focusing on high-grade serous ovarian carcinoma (HGSOC). Leveraging the growing understanding that HGSOC originates from precursor lesions in the fallopian tube, the researchers examined EVs derived from murine fallopian tube (mFT) cells with oncogenic mutations and identified candidate markers specific to fallopian tube origins. In a pilot study involving human patients, these EV markers were validated for their high accuracy in distinguishing HGSOC from non-cancer cases. This innovative approach would offer a minimally invasive method to monitor women at high risk of ovarian cancer, facilitating timely intervention strategies. Learn more...
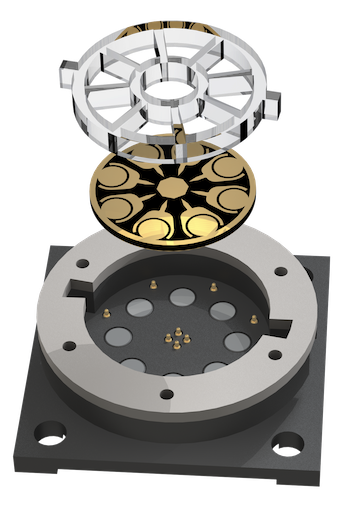
Electrical engineering to boost electrochemiluminescence
Electrochemiluminescence (ECL) is a powerful biosensing method with inherently low background and precise chemical reactions through electrical control. In recent work published in Science Advances, CSB investigators extended ECL’s advantages by devising a new system termed ECLipse (ECL in paired signal electrode). The key innovation was to separate ECL generation from target detection: these two processes were carried out in isolated chambers and coupled through an electrode. With this strategy, the investigators could maximize the analytical signal without crosstalk and integrate multiple sensors in a compact chip. In a pilot clinical study, the ECLipse system accurately detected septic conditions by measuring host factors (IL-3, IL-6, PCT) and revealed distinct IL-3 and IL-6 patterns in patients with SARS-CoV-2 infection. Learn more...