Powerful new test allows a glimpse into tumor dynamics
Cellular cancer diagnostics are essential in establishing the correct diagnosis, choosing the appropriate treatment, assessing therapeutic efficacy and in better understanding the tumor microenvironment in clinical samples. Current clinical workflows are largely focused on obtaining a tissue core biopsy taken at the beginning of a treatment. Cancers, however, are highly dynamic under treatment pressures and the inability to serially and rapidly assess the tumor microenvironment (TME) hampers our ability to rationally adjust treatments without delays and understand the underlying biology. Furthermore, core tissue biopsies have a considerable procedural morbidity (16-18G core needles are used for tissue harvesting), are costly (deep sedation, physiologic monitoring), not infallible (insufficient samples in up to 20-30%) and time-consuming (days to weeks from acquisition to results). In contradistinction, fine needle aspiration (FNA) using “skinny needles” yields cells rather than tissue and could circumvent these practical bottlenecks and enable serial profiling. Unfortunately, existing cellular analytical methods are often rather limited by the number of markers (conventional cytopathology), are non-representative of the TME (flow cytometry of immune cell populations in peripheral blood), or are limited by very high costs and long analytical times (scRNA-seq), i.e. not practical for routine clinical use.
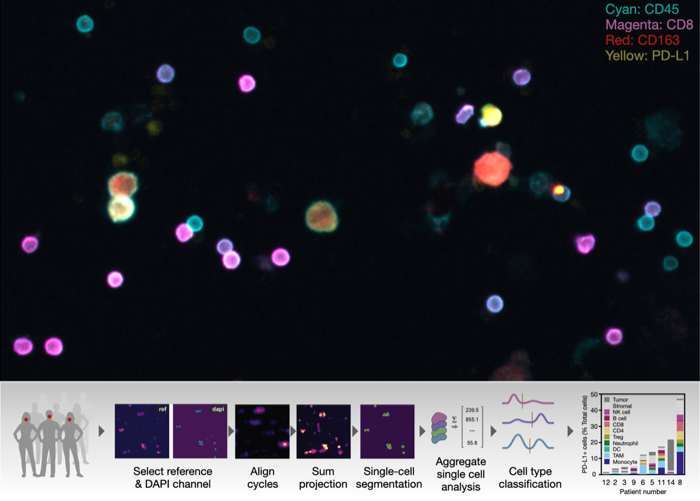
Here, researchers at CSB report on the development, testing and validation of a novel multiplexed FNA assay (FAST-FNA) to measure dozens of tumor and immune cell markers on individual cells. The method relies on newly developed bio-orthogonal cycling reagents that allow de-staining of fluorescent labels within seconds while still preserving the integrity of cells.
Thus, it is possible to image 20-40 molecular markers per cell in pauci-cellular FNA samples and provide comprehensive TME analysis within the same day of aspiration. With this approach, the team was able to track the temporal changes within the TME with anti-PD1 therapy in preclinical tumor models. Furthermore, FNA samples from patients with head and neck squamous cell carcinoma (HNSCC) were investigated. The team reports that PD-L1 scoring on tumor and immune cells in the TME using the FAST-FNA assay is comparable to the gold standard of Combined Positive Score (CPS) on whole tumor tissue section, but can now be done on cells and reported on the same day. Since FAST-FNA covers a broad immune cell repertoire, it also has the potential to lead to new and better predictive biomarkers of immunotherapy. Lastly, they demonstrate the feasibility of performing serial FNA analysis to track changes in the TME during the course of immunotherapy in HNSCC patients. The scalable and versatile nature of FAST-FNA assay allows its application to other clinical situations where rapid answers are desirable and/or when serial tissue sampling can inform drug efficacy and/or clinical decision making.