The spleen’s newly discovered function
In a ground-breaking study published in Science 2009, researchers at the Center for Systems Biology (CSB) showed that the spleen, an organ previously regarded as somewhat redundant, in fact plays a key role in the healing response following a heart attack. As it happens, the spleen functions as a reservoir for specific immune cells, known as monocytes. Up until this discovery, the bone marrow was believed to be the sole source of these cells, not only producing them, but also releasing them into the blood stream to circulate the body and respond to signs of injury or inflammation. The investigation published in Science, however, was the first to show that following injury to the heart, it is the spleen-derived monocytes, rather than those in circulation, that play the major role in repair.
This important study was the result of a close collaboration between different CSB investigators, namely Mikael Pittet, Fil Swirski, Matthias Nahrendorf and Ralph Weissleder. Here, Mikael Pittet, senior author on the 2009 Science article, discusses this work.
How did this study unfold?
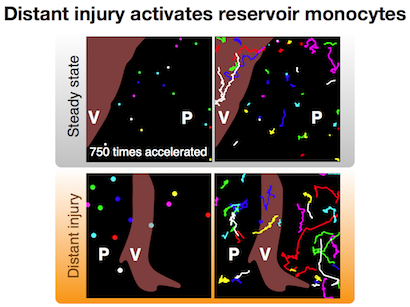
MP: We wanted to look at the monocyte/macrophage response following myocardial infarction. However, what we found was that the number of cells recruited to the site of injury was surprisingly high. It was actually much higher than is readily available in the blood. So, because the numbers did not match, we thought it important to know where these cells come from.
Initially, an obvious explanation was the bone marrow, since we know that the bone marrow generates monocyte precursors. We therefore thought that may be the bone marrow somehow rapidly releases these cells into the blood, which then go to the heart and help repair injured tissue. But this was not the case.
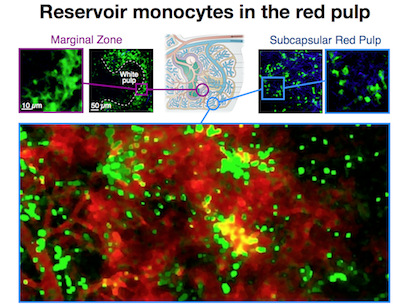
We then decided to screen for all the tissues that could produce these cells, and found that the spleen was one organ that actually incorporates a reservoir of monocytes. This was very surprising, however, since by definition a monocyte is a cell that is ‘in circulation’, and at the moment that it enters a tissue, it differentiates into what is known as a macrophage. Other investigators had previously found these cells in the spleen but defined them as as macrophages not monocytes. So what we wanted to do was determine whether these were really monocytes; and if they are monocytes, can they egress from this reservoir site and relocate to an injury site?
…and what was the conclusion?
MP: Basically, our study showed that this is indeed the case. The spleen contributes a very high fraction of monocytes, which are very important for repairing injury to the heart.
What would say are the implications of this finding?
MP: The immediate significance is the fact that it is always pretty cool to identify a new function for an organ: the spleen now turns out to act as a reservoir for monocytes. No-one knew about this before.
Long term, however, it would be useful to know whether this reservoir is really that important. In a mouse model of heart attack, it appears to be very important but we don’t know whether it is important in other diseases or whether it is important in humans. So we would like to expand this research to different diseases, and to humans.
Would you speculate that the spleen is generally useful for proper healing?
MP: Well it depends. If you have a tissue injury, it would be useful to have this surge of monocytes that go to the site of injury and repair damage. But in cancer, you don’t want this, because the monocytes could get corrupted by tumor cells and actually promote inflammation, tumor growth and metastasis.
Knowing about this monocyte reservoir, could it now be somehow exploited to help treatment?

MP: Yes. That is what we are now interested in doing, but we don’t yet know whether we should selectively look at the spleen or whether we should look more generally. It is possible that the spleen may not be a unique source of these cells; different tissues may produce or release other cells that are also very important. Ultimately, this means that when we want to tailor immune responses for therapeutic benefit, we will need to take all these considerations into account.
Are you following this work up?
MP: Yes, we are following this up on several levels. Right now my lab is focused on trying to find out whether these cells are important in cancer. Also, in collaboration with the Weissleder lab, we are looking to see what effect inhibiting these cells with drugs has. Fil Swirski’s lab is currently exploring the involvement of these cells in the context of sepsis and bacterial infections, whereas the Nahrendorf lab is primarily interested in the role of these cells in cardiovascular biology.
Written by Yvonna Fisher-Jeffes, PhD
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ
Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites
Science. 2009;325 (5940):612-6 – PMID: 19644120 – PMCID: PMC2803111