Local intervention for atherosclerosis could be on the horizon
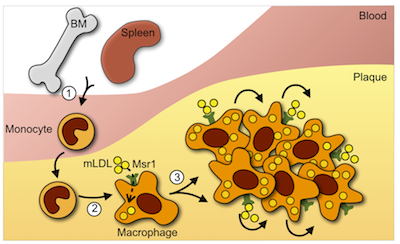
New insights into how vulnerable atherosclerotic plaques develop could soon lead to better treatment or prevention of heart attacks and strokes. Inflammatory cells have long been known to play a key role in atherosclerosis, but despite many advances in our understanding of cardiovascular disease, the precise pathobiology of plaques has continued to elude investigators. Now a pioneering study by researchers at the Center for Systems Biology (CSB) has taken the bold step of re-evaluating previous assumptions by showing that inflammatory plaques do not exclusively rely on recruitment of circulating immune cells from the blood, as presumed, but can in fact grow from within. Indeed, the dominant mechanism for plaque development appears to be proliferation of plaque-resident macrophages. The findings are reported online in the August 11th issue of Nature Medicine.
Atherosclerotic plaques are a conglomeration of inflammatory cells, fats, cholesterol and other substances. Until recently, the prevailing theory of atherosclerosis was that plaques grow by drawing white blood cells, known as monocytes, in from the blood. These monocytes, in turn, mature into lipid-munching macrophages, which gorge themselves, get stuck, and consequently contribute to the volatility of the plaque’s fatty ‘core’. Rupture of these plaques leads to clot formation, which can block blood flow to the heart or brain causing a heart attack or stroke. The holy grail of cardiovascular research has therefore been to find a way to slow or stop plaque development.
The vulnerability of plaques to rupture is closely linked to the quantity of inflammatory macrophages. Macrophages are phagocytic ‘garbage disposal’ cells, typically seen in injured or diseased tissues. They are derived from circulating blood monocytes, which mature into macrophages upon entry into tissue. The theory to date has therefore argued that the large numbers of macrophages seen in vulnerable lesions are the result of monocytes being recruited from the circulation. This theory, however, has now been called into question by the very same research team that were originally one of its major supporters.
A dynamic environment
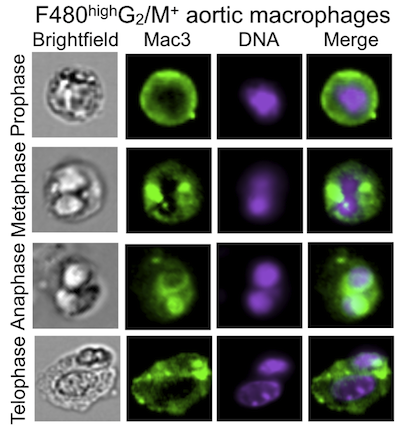
“What we discovered is that the atherosclerotic lesion is a very dynamic environment,” explains Filip Swirski, Assistant Professor at the Center for Systems Biology and senior author on the paper, “and one that does not exclusively rely on monocyte influx”. In their study, the CSB researchers used nucleotide (DNA) tags in order to detect cell division within the plaques of experimental mice, and then depleted circulating monocytes via drug-releasing implants. “Even in the absence of monocytes, we were surprised to see continuous renewal of macrophages in lesions,” says Swirski. “So we wanted to know where they come from.”
A series of very elaborate and sophisticated experiments were thus designed to determine the relationship between blood monocytes and tissue macrophages in more detail. Among them, parabiosis—a procedure involving the reversible joining of two experimental animals—was employed to investigate the effects of circulating monocytes from one co-joined animal on plaque formation in its monocyte-depleted partner. “Ultimately, we showed, through the use of many techniques, that local macrophage proliferation is the dominate mechanism underlying macrophage accumulation in established disease” says Swirski. “Even though lesional macrophages are fundamentally derived from monocytes, lesions do not require constant monocyte input to sustain their macrophage numbers”.
The team also shed light on the molecular mechanism involved. Namely, that macrophage turnover relies on the macrophage-expressed scavenger receptor A (SRA), a process that is thought be entirely independent of the mechanism underlying monocyte recruitment.
Major re-evaluations
In treating atherosclerosis, the goal is primarily to ensure that lesions remain stable and at low risk for rupture. “Currently, there is quite a bit of interest in targeting inflammation as a way to treat vascular disease” says Swirski. “And one of the ways that you can target inflammatory processes is by targeting the cells responsible.”
In view of the latest findings that, besides monocyte recruitment, macrophage self-renewal is a major component of atherosclerosis, it is possible that local inhibition of macrophage proliferation could prove an effective therapeutic intervention in the future.
“I think this work will force some major re-evaluations,” says Swirski. “People have been thinking of targeting monocyte influx, but what they need to consider is macrophage proliferation as an additional or alternative approach, especially in established disease.”
“This might actually be much better than targeting circulating monocytes,” concludes Swirski, “since interrupting pathological processes locally within the plaques themselves would spare other beneficial host responses mediated by these circulating monocytes.”
Written by Yvonna Fisher-Jeffes, PhD
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, Rooijen NV, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK
Local proliferation dominates lesional macrophage accumulation in atherosclerosis
Nature Med. 2013;19(9):1166-72 – PMID: 23933982 – PMCID: PMC3769444
Press Coverage
Futurity – Plaque May Work From Inside To Clog Arteries (pdf)
Science Daily – New Study Redefines How Plaques Grow in Heart Disease (pdf)
Medical News Today – Heart disease plaques: ‘theory-changing’ discovery (pdf)
HealthCanal – New study redefines how plaques grow in heart disease (pdf)
Medical Xpress – Macrophage proliferation appears to drive progression of atherosclerosis (pdf)
Science Newsline – New Study Redefines How Plaques Grow in Heart Disease (pdf)
News Daily – Local macrophage proliferation may play key role in atherosclerosis (pdf)
The Huffington Post – New Understanding Of Atherosclerosis Could Change The Way We Treat Heart Disease (pdf)
ANI News – New twist to how ‘deadly’ plaques grow in heart diseases (pdf)
Angina.com – Atherosclerosis: Macrophages can Proliferate Locally Within the Plaque. Interview with: Clint S. Robbins, PhD (pdf)