Novel therapy turns off navigation system in disease-promoting cells
During illness or injury, distressed cells release a cocktail of chemicals that attract immune cells to the damaged site. Like a chemical GPS, immune cells follow these signals, which navigate them to the right place. Researchers at MGH, together with collaborators at MIT and Alnylam Pharmaceuticals, have now developed a way to manipulate this navigation system by selectively silencing the protein that inflammatory—but not reparative—cells use to detect these signals. In so doing, inflammatory cells, which are often propagators of disease, are rendered “blind” to sites of injury and thus tissue damage is reduced rather than exacerbated. Preliminary studies with this novel anti-inflammatory ‘treatment’ have shown that it has therapeutic effects in a wide range of diseases including cancer, cardiovascular disease and transplant rejection. The findings are published in the October issue of Nature Biotechnology.
Too much of a good thing
In the body, white blood cells known as monocytes are critical to the immune response. “In good times, these cells defend, eat bacteria, and promote early stages of wound healing,” explains Matthias Nahrendorf, senior author of the paper. “But in disease, they can be harmful.”
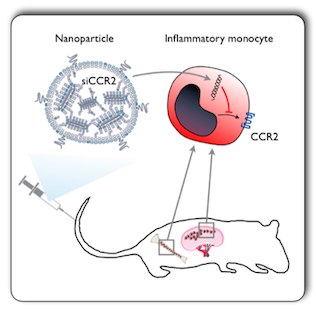
Monocytes can be broadly categorized into two subsets: an inflammatory subset and a non-inflammatory subset. The former is normally required for defense and for removal of debris, but too many of these cells can result in the propagation of disease. The latter subset of monocytes are slower to come on the scene and appear to have a more reparative role. Interestingly, each subset employs a unique chemical navigation method to migrate to sites of injury. “The bad guys—the inflammatory subset—use the receptor CCR2,” explains Nahrendorf, who is also an Assistant Professor of Radiology at the Center for Systems Biology.
Monocytes expressing the CCR2 receptor are attracted to sites of injury, infection and inflammation via the signaling protein MCP1, which is released by damaged cells. Because monocyte recruitment more often than not has a negative effect, the research team at MGH wanted to find a way to stop them from migrating. In collaboration with researchers from MIT and Alnylam Pharmaceuticals, they subsequently devised a strategy that identifies and blocks production of the CCR2 receptor on monocytes such that these cells are rendered “blind” to MCP1, and thus unable to migrate towards it.
Silencing CCR2 production
In cells, DNA code is translated into functioning proteins with the help of messenger RNA. As the name suggests, messenger RNA (or mRNA) is responsible for copying protein-coding portions of DNA and carrying the message from the nucleus to the cytoplasm. Once there, it travels to the ribosomes—the protein-making factories of the cell—to be translated into functioning biological molecules.
This system can be neutralized with a molecule known as ‘small interfering’ RNA (siRNA), which essentially binds to its target mRNA, degrades it, and ultimately prevents the protein it codes for from being produced.
In recent years, exploiting this system for therapeutic intervention has become
increasingly attractive. “The exciting thing about siRNAs is that you can selectively ‘silence’ any single protein,” says Nahrendorf. “In this specific case, the protein we selected, CCR2, has big therapeutic implications. It has the potential to be a potent anti-inflammatory treatment.”
However, according to Nahrendorf, one of the hardest things in using siRNA to silence protein expression is delivering the siRNA to the right place. “It not only needs to go to the cell of interest but it needs to reach the right compartment within that cell of interest.” To do this, packaging the right siRNA sequence in the right delivery vehicle is critical.
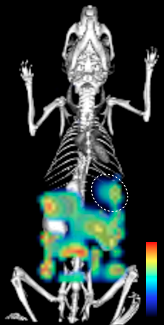
By collaborating with investigators at Alnylam Pharmaceuticals, a biopharmaceutical leader in RNA interference-based therapeutics, the MGH researchers were able to obtain promising siRNA sequences against CCR2. Once the top siRNA sequence had been identified, it was labeled with a fluorescent tag. The molecule was then encapsulated into a specifically-designed nanoparticle delivery system developed by collaborators, Robert Langer and Daniel Anderson, at MIT. This preparation was then injected into mice and thus, via optical imaging that picks up the signal given off by the fluorescent tags, the investigators could see whether the siRNA reached target monocytes, and into which tissues it localized.
However, as Nahrendorf explains “it is not enough for the siRNA to simply localize to the target cells, it needs to be in the cytoplasm where the mRNA [coding for CCR2] is”. To check that the siRNA was indeed going to the right place and blocking CCR2 production, the investigators did two things. Firstly, they looked at the CCR2 protein levels in monocytes to confirm that they had gone down, and secondly, they looked for specific cleavage products. “When siRNA binds to your mRNA target, the mRNA gets cleaved,” explains Nahrendorf, “and you can detect those cleaved products with a specific assay.”
Once verified that CCR2 production in these cells was being blocked, the investigators then isolated monocytes from mice who had received the siRNA preparation and, using an in vitro assay, looked at their ability to detect and travel towards MCP1. “And we found that they couldn’t,” says Nahrendorf. “When production of CCR2 was shut down, these monocytes were not able to migrate towards MCP1.”
Wide-ranging therapeutic effects
“The interesting thing is that these inflammatory monocytes are involved in almost all major diseases you can think of,” says Nahrendorf.
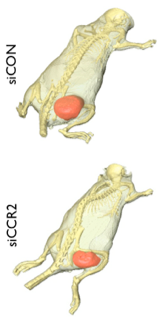
Based on this knowledge, the research team subsequently tested the therapeutic effect of blocking CCR2 production in a variety of sophisticated animal models, namely atherosclerosis, heart failure, cancer and transplant rejection. Impressively, the siRNA preparation was effective in all of them with “the number of monocytes in all of these models reduced anywhere from 50 to 80%.”
The investigators likewise saw a corresponding reduction in disease pathology. “For example,” says Nahrendorf, “tumors in mice weren’t growing as fast, infarct sizes in mice with myocardial infarction were smaller, and atherosclerotic plaques were smaller and less inflamed.”
According to Nahrendorf, “the problem with anti-inflammatory drugs currently on the market is you hit all cells all over the body; this can result in effects you don’t want. With this new siRNA anti-inflammatory treatment, non-inflammatory cells that don’t rely on the CCR2 receptor are not affected. That’s the big deal.”
The research group is currently working on optimizing this preparation to determine how best it can be used. They are also examining the therapeutic effects of other siRNA preparations and likewise whether simultaneous targeting of several proteins could enhance therapeutic efficacy further.
Written by Yvonna Fisher-Jeffes, PhD
Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M
Therapeutic siRNA silencing in inflammatory monocytes in mice
Nat Biotechnol. 2011;29(11):1005-10 – PMID: 21983520 – PMCID: PMC3212614