Finding a needle in a haystack
Tumors can shed cells into the bloodstream that circulate throughout the body. These circulating tumor cells, or CTCs, have been thought to play an important role in the spread of cancer. However, because these cells are extremely rare, the detection of CTCs for use as a cancer diagnostic has proven highly challenging. Typically, there are only 1-10 CTCs per milliliter of blood. Considering there are around 5 billion red blood cells, 10 million white blood cells and 300 million platelets in the same volume, you can understand why finding CTCs has been likened to finding a needle in a haystack. But like the needle, it appears that the solution to finding these rare cells may be the same: use a magnet (or in the case of CTCs, a nano-magnet). And this is precisely what researchers at the Center for Systems Biology (CSB) have done. A novel microchip was developed that can rapidly scan through the enormous number of blood cells to find these very rare CTCs, using a combination of microelectronics, microfluidics, and nanotechnology. This new system, described in the July issue of Science Translational Medicine, holds the potential for accurate on-the-spot cancer diagnosis and treatment monitoring.
Magnetic detection
“One of the big challenges of this work,” explains David Issadore, a post-doctoral fellow at the CSB and first author of the study, “is that because CTCs are so rare, there is not a reliable ‘gold standard’ technique for detecting them.” Most forms of cell detection—using fluorescent labels for example—are not powerful enough to identify such rare cells, since background noise from surrounding tissue can drown out any signal emanating from these cells. Magnetic detection is ideally suited to this problem because biological tissue is inherently not magnetic and thus any signal obtained will be from the magnetically tagged cells.
To identify tumor cells using magnetic sensing, molecular probes are used to identify proteins on the surface of cells which are associated with tumor cells. These molecularly labeled cells are subsequently tagged with specially designed magnetic nanoparticles, and a magnetic detector can then be used to measure the presence of these magnetic tags within a sample.
The trouble is that, while magnetic detection gets around the problem of background interference, it can still suffer from a lack of specificity. “Some of the magnetic nanoparticles end up on other cells, besides the tumor cells,” says Issadore. “So the overall magnetic signal from the sample can come largely from these background cells, especially in the case of CTCs where the tumor cells are greatly outnumbered by blood cells.”
Using the Hall effect to detect cells
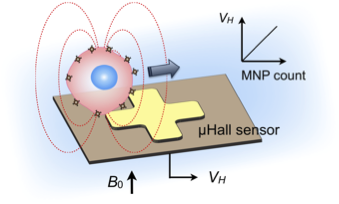
To overcome this problem, CSB researchers have now come up with a new system, which, instead of measuring all of the cells in a sample together, measures each cell individually.
The device is based on microelectronic chips, which use magnetic Hall effect sensors the size of an individual cell, to interrogate the number of nanoparticles per cell. As in previous forms of magnetic detection, samples are mixed with a cocktail of antibodies that bind to tumor-specific proteins expressed on the cells, and these are then labeled with magnetic nanoparticles.
What makes the device unique, however, is that samples are injected into a microfluidic channel that is positioned directly above an array of miniaturized Hall sensors. Thus, as the sample flows through the channel, the magnetic field of each passing cell can be rapidly measured by the underlying sensors. Accordingly, cells with large numbers of tumor-specific proteins attract more magnetic nanoparticles to their surface and when these highly magnetic cells pass the miniaturized Hall sensors, there is a measurable change in signal.

Detecting Rare cells
The Hall system took almost three years to develop. “Combining the microfluidics with the Hall sensors was very challenging,” explains Jaehoon Chung, an author on the paper and post-doctoral fellow responsible for developing the microfluidic components for the Hall system. Once optimized, however, the device was shown capable of detecting tumor markers with high accuracy both in cultured cell samples as well as in whole blood spiked with known quantities of tumor cells. In both cases, it demonstrated analytical power on the same level of current standard lab techniques. But the point, according to Issadore, was to detect cells that were hard to spot using conventional methods.
The research group thus tested the device on whole blood samples from patients with late stage ovarian cancer who, because of their advanced disease, were more likely to have higher levels of CTCs. Results were then compared with those obtained using CellSearch, the only currently approved technology for detecting CTCs.
“It worked really well,” confirms Issadore. Findings with the Hall sensor showed that CTCs could be detected in 96% of patient samples whereas CellSearch could only detect tumor cells in 15%. “The Hall sensor’s superior performance is likely in part thanks to the fact that the device can detect rare cells directly in whole blood,” explains Issadore. “With CellSearch, samples need to be processed, which leads to cells being lost.” The other reason why the Hall sensor worked better is likely because it detects four different tumor-specific proteins, whereas CellSearch only detects one protein; since CTCs are not so simple as to be identifiable from a single marker, many cells can go undetected.
Finally, to show that the Hall sensor could also be used to monitor treatment efficacy, the research group performed a mouse study in which the sensor was used to detect therapy-related changes in cancer markers. The results showed that not only did the Hall sensor detect therapy-related changes in cancer protein levels but that decreases correlated with reductions in tumor size.
An App for that?
According to Hakho Lee, co-senior author of the paper, this new system is now poised to enable on-the-spot cancer diagnosis and monitoring. He says, “improving the electronics and microfluidics, is one of our top priorities. This will increase the reliability of the device and will make the device more user-friendly for on-site use in the clinic.”
Indeed, their ultimate goal is to develop an App for use with smart-phones.
The group are also currently adapting the device to other biological targets such as TB bacteria, which would be of enormous benefit to the developing world.
Written by Yvonna Fisher-Jeffes, PhD
Issadore D, Chung J, Shao H, Liong M, Ghazani AA, Castro CM, Weissleder R, Lee H
Ultrasensitive Clinical Enumeration of Rare Cells ex Vivo Using a Micro-Hall Detector
Sci Transl Med. 2012;4 (141):141ra92 – PMID: 22764208 – PMCID: PMC3603277 – Cover