Glow-in-the-dark plaques
Heart attacks and strokes are dubbed the “silent killers” of society due to their sudden onset and the difficulty in predicting them. As a result, they remain a leading cause of death, with over 2000 Americans dying of cardiovascular disease a day. While effective treatment options have evolved over the last 35 years, our ability to reliably detect and monitor the molecular changes underlying cardiovascular disease are still limited. However, this is now poised to change. Researchers at the Massachusetts General Hospital (MGH) have made huge advances toward the clinic by showing that a fluorescent imaging agent, already approved for humans, can reliably identify inflamed fatty plaques in blood vessels the size of human coronary arteries. Occlusion or even rupture of such deposits is the primary cause for heart attack and stroke and thus having the ability to detect them could revolutionize cardiovascular diagnosis. The team report their findings in the June issue of Science Translational Medicine.
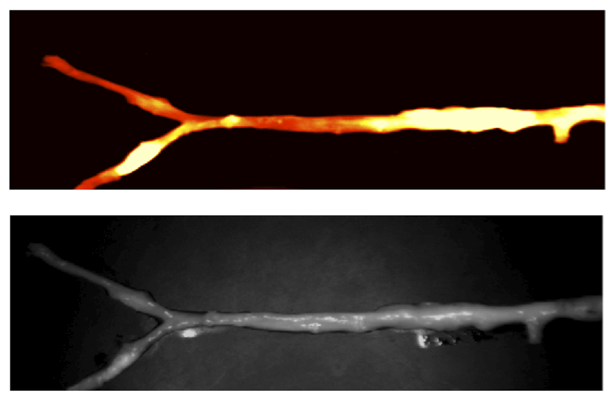
Detecting vulnerable plaques
Fatty deposits, known as plaques, gradually build up on the inside of arterial walls over many years and cause chronic inflammation. Left unchecked, they can lead to cardiovascular disease either because they grow so big as to occlude blood flow, or because they rupture and cause a clot that results in blockage. Contrary to popular belief, obstructions due to large plaques only account for a small percentage of heart attacks or strokes. The majority are actually caused by rupture of inflamed fatty plaques, where infiltration of immune cells renders the plaque particularly unstable and “vulnerable” to rupture. While techniques for detecting arterial plaques exist, they are not yet sophisticated enough to determine whether a particular plaque is at risk for rupture or not. Furthermore, the problem with vulnerable plaques is that since they do not necessarily bulge out into vessels they often elude typical detection tests, and so even finding them can be a challenge.
Arterial plaques are currently detected using an X-ray based imaging procedure known as angiography. A catheter is inserted into an artery (typically in the forearm or groin) and then threaded through the blood vessel until it reaches the area to be imaged. A special fluid or “contrast agent” is subsequently injected through the catheter, and its passage through the blood vessel can be picked up on X-ray. The presence of plaques are identified by areas of vessel narrowing.
“This [technique] has been around for over 50 years,” explains Ralph Weissleder, an author on the paper and Interventional Radiologist as well as Director of the Center for Systems Biology (CSB) at MGH. The problem with it is you don’t know what’s happening in the vessel wall, whether there is active inflammation or not."
Other imaging techniques such as positron emission tomography (PET) and magnetic resonance imaging (MRI) are able to provide more detailed information. But their resolution is only sufficient for detecting large plaques in large vessels. They are also more time consuming and still require therapeutic intervention.
Glow-in-the-dark plaques
A few years ago, to address the problem of detecting vulnerable plaques, MGH researchers designed a new optical imaging device capable of detecting plaque inflammation in rabbits (Jaffer et al. Circulation 2008). This minimally invasive system involved injection of a fluorescent “glow-in-the-dark” contrast agent, which when activated by a fiber optic probe inserted via catheter into the blood vessel, could highlight areas of inflammation. The only problem here was that the fluorescent agent used was only approved for use in mice or rabbits, and not for humans. Moreover, it needed to be administered 24 hours before imaging could take place. Thus, to enable this device to reach the clinic, a faster acting clinically approved agent needed to be found.
A new use for an old agent
A promising candidate that emerged was the fluorescent imaging agent indocyanine green (or ICG).
“ICG was already clinically approved,” explains Claudio Vinegoni, an Instructor at the CSB and first author of the paper. “It was already known to bind lipids. So because when you have inflammatory plaques, you have areas rich in both fat and immune cells, we decided to see whether ICG could bind plaques.”
The research group led by senior author Farouc Jaffer, Assistant Professor of Medicine at MGH and an Interventional Cardiologist, who was also once a postdoctoral fellowship in the Weissleder group, used an animal model of plaque formation. Rabbits on a high cholesterol diet each received a small arterial injury, which over a period of several weeks developed into inflammatory plaques. Rabbits were then injected with ICG, and its distribution was visualized using the novel optical imaging system developed by the research group. As in their previous study, a catheter containing an optical fiber was inserted into the rabbits’ artery. Like a glow-in-the-dark toy, upon excitation by a laser beam (also in the catheter), ICG immediately produced a fluorescent light signal. This light was then detected by the optical fiber, which transferred it to an external sensing device. In this way, the extent of plaque inflammation, and thus its “vulnerability” for rupture, could be quantitatively assessed; namely, the brighter the glow, the quicker to blow!
To subsequently verify that the optical imaging of ICG was indeed detecting plaques, the researchers performed angiograms to identify areas of vessel narrowing, as well as ultrasound scans within the vessels to examine their inner surface. “Of course, these techniques can’t tell if these plaques are inflammatory,” says Vinegoni. “This information could only be determined by ICG imaging.”
Once imaging was done, histological analyses of the blood vessels confirmed that ICG was, as predicted, taken up by the fatty plaques. Some ICG even appeared to be internalized by specific immune cells known as macrophages, which infiltrate the plaque.
However, while these findings clearly demonstrated that ICG is taken up by inflamed plaques in rabbits, there was no evidence to show that the same is true for humans. Thus, for the final part of the study, the uptake of ICG was investigated in sections of human carotid plaque. These sections were incubated with ICG, whose distribution was then determined by imaging and histology. “Our results were similar to those seen in rabbits,” says Vinegoni. “We saw both binding by lipids and uptake by macrophages.” However, Vinegoni was quick to emphasize that this human study was preliminary and whether the same effect occurs within the human body, can only be determined by performing clinical trials.
Nevertheless, according to Vinegoni, the implications for these findings are “huge”. “First of all, ICG is already FDA approved; this means we could use it in patients tomorrow.” The other key thing according to Vinegoni is its fast uptake into plaques. “It means that if a patient came into the cardiac catheterization laboratory, you could inject the agent, and in the time it takes to prepare everything, you could already start imaging.” Dr. Jaffer says the next step is to obtain clinical approval for their catheter-based imaging system as well as approval for the use of ICG to image human arterial plaques.
Written by Yvonna Fisher-Jeffes, PhD
Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, Ntziachristos V, Weissleder R, Libby P, Jaffer FA
Indocyanine Green Enables Near-Infrared Fluorescence Imaging of Lipid-Rich, Inflamed Atherosclerotic Plaques
Sci Transl Med. 2011;3 (84) ra45):84ra45 – PMID: 21613624 – PMCID: PMC3112179
Press Coverage
voanews.com
netdoktor.de
astrazeneca.at
thenakedscientists.com
newspaperstoday.com
blogs.nature.com
excitonic.com
incirculation.net
medwirenews.com
elmundo.es