Imaging the beating heart in the living body
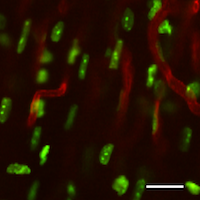
Imaging the beating heart within the body has been difficult, mostly because the high frequency with which the cardiac muscle pumps means that it is rarely still long enough for current microscopes to take accurate images. Recently, however, a new imaging method developed by researchers at the Center for System Biology has been shown capable of generating real-time images of the living heart. It is reported in the October issue of Nature Communications.
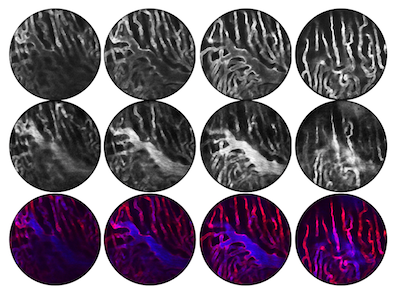
Imaging living organs within the body in real-time has always been a challenge. To avoid significant motion artifacts, current technology requires objects to remain stationary. Breathing and cardiovascular movements, however, tend to reverberate throughout the body, which in turn perturb every organ. Thus, imaging of such organs is impossible without controlling motion. Whilst a number of motion suppression techniques have been developed to image organs in the past, they have only been suitable for use on organs that either move slowly or that have a small range of motion. Typically, these strategies have involved compression. “But this doesn’t work for an organ such as the heart,” explains Claudio Vinegoni, Associate Professor at the CSB and co-first author of the report, “because you will not only reduce the amount the heart is free to pump but you will also compromise its integrity.” As a result, knowledge of the heart’s natural physiology and function within the living body has been limited. To address this, the research group at the CSB devised a new method for acquiring stabilized images. “Our objective was to show that we can image the beating heart at sub-cellular resolution in real-time,” says Vinegoni. In their study, the investigators demonstrated their novel method by imaging ischemia in the heart of an anethetised mouse. A ventilator was used to control breathing motion and a stabilizing ring was adhered to the heart to minimize cardiac movements. To reduce motion artifacts even further, an optimal imaging window, where both breathing and cardiac movements were minimal, was then identified. “We found that we obtained maximum stabilization at the point where the end of expiration coincided with the end of the diastole phase of the cardiac cycle,” said Vinegoni. “We therefore acquired data only at this specific time point and then produced images by patching together all the information gathered during these periods”. Ultimately, by combining this ‘gating’ technique together with the stabilizing ring, the researchers were able to visualize the recruitment of white blood cells to areas of damage in the heart in real-time over several hours. Indeed, the set-up was even capable of repeat imaging over several days.
“In the paper, we demonstrate the potential of the technique,” says Vinegoni, “which is to track cells in real-time. It will now be very interesting to use this method to study what happens immediately after re-perfusion of the heart following an ischemic attack. Nobody knows what happens at the microscopic level in the region of the infarct.” Vinegoni believes that the strategy will provide invaluable biological insight since it allows the recruitment of cells, the formation of collagen as well as changes in the contraction of heart cells to be visualized in real-time. The technique is also easily adaptable to the imaging of other organs.
Written by Yvonna Fisher-Jeffes, PhD
Lee S, Vinegoni C, Feruglio PF, Fexon L, Gorbatov R, Pivovarov M, Sbarbati A, Nahrendorf M, Weissleder R
Real-time in vivo imaging of the beating mouse heart at microscopic resolution
Nat Commun. 2012;3:1054 – PMID: 22968700 – PMCID: PMC3622400