Not just a ‘plumbing’ disease
New research at the Center for Systems Biology (CSB) has shown that heart attacks are not just a “plumbing” problem in the arteries but a ‘whole system’ condition, resulting in widespread inflammation and a predisposition for a secondary attack. Findings are reported in a Nature article, which is receiving advanced online publication on June 27, 2012.
Heart attacks (myocardial infarction) result when one of the arteries feeding the heart becomes blocked, leading to death of a portion of the heart muscle. They are most commonly the result of atherosclerosis, which is the build up of hard fatty deposits, known as plaques, on the inside of arteries. Typically, large clots, following the rupture of these plaques, are the most frequent causes of infarction.
“Once you have an infarct, the likelihood that you will get another one is very high,” explains Matthias Nahrendorf, co-senior author on the paper with Ralph Weissleder, Director of the CSB. “So we did this study because the frequency of myocardial infarction made us think that may be the infarct does something to the whole system.”
The team was already aware that myocardial injury results in a systems-wide response, after previous research at the Center showed that following myocardial injury, production of a certain type of inflammatory cell is stimulated in the spleen. These cells, known as monocytes, then travel to the site of injury, where they are involved in healing.
The team’s latest research, however, now adds another piece to the puzzle by showing myocardial infarction leads to an acceleration in atherosclerosis, where monocytes are a major driving force behind growth and inflammation of plaques. In their study, they showed that in contrast to normal atherosclerotic mice fed a high fat diet, plaque inflammation in atherosclerotic mice following an infarct is dramatically increased. “Their plaques were bigger, more inflamed and more dangerous,” says Nahrendorf, who is also Assistant Professor of Radiology at the CSB.
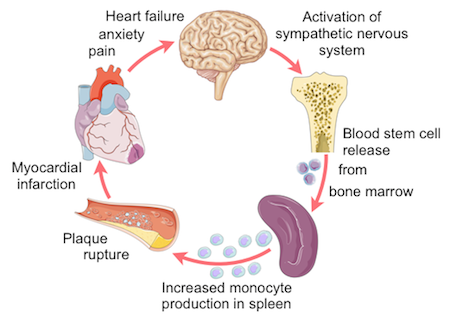
As a consequence of this finding, the team then performed a series of additional experiments, including flow cytometry assays, drug studies and imaging, to look into how this might come about. Based on their results and on clinical reports, the investigators discovered a new feed back loop in which the pain and anxiety often reported by patients following an infarction acts to stimulate the sympathetic nervous system. In turn, this stress signal triggers blood stem cell release from the bone marrow and inflammatory cell production in the spleen, which starts producing surplus amounts of monocytes. Greater numbers of these cells then enter the circulation and are recruited to plaques, which leads to their increased vulnerability, and ultimately a higher chance of a second infarct.
“I hope these new findings will widen the view and encourage researchers in the field to start thinking about the system as a whole rather than assuming that atherosclerosis is a local disease,” says Nahrendorf. “Knowing about this inflammatory process is useful because we can now think about therapies to stop this positive feedback loop.”
Written by Yvonna Fisher-Jeffes, PhD
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AN, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M
Myocardial infarction accelerates atherosclerosis
Nature. 2012;487(7407):325-9 – PMID: 22763456 – PMCID: PMC3401326
Press Coverage
Boston Globe – Body’s own immune response may accelerate heart attack recurrence (pdf)
Mass General Hospital – Immune response to heart attack worsens atherosclerosis, increases future risk (pdf)
Nature News&Views – Cardiology: Bad matters made worse (pdf)
Frankfurter Rundschau – How the mind controls the heart (pdf)