New assay platforms for molecular profiling of extracellular vesicles (EVs)

My laboratory has been pioneering a repertoire of new assay technologies tailored for EV for analyses (details can be found in our extensive review article). These include i) acoustic-wave nanofilter for EV isolation in blood; ii) new electronic or optical sensors (microNMR, nanoplasmonic chip) for EV protein screening; iii) integrated microfluidics for EV RNA analysis; and iv) a new imaging platform for single EV profiling. Our clinical research has established EVs’ utility in cancer diagnosis and treatment monitoring. We found i) high correlation between EV protein profiles and disease progression, and ii) potential of EV mRNA screening to predict treatment responses. Our flagship technology, nPLEX (nano-plasmonic exosome) was selected as one of the greatest hits among 20 years of Nature Biotechnology biomedical research. nPLEX is now being tested for early cancer detection.
Computational imaging and machine learning
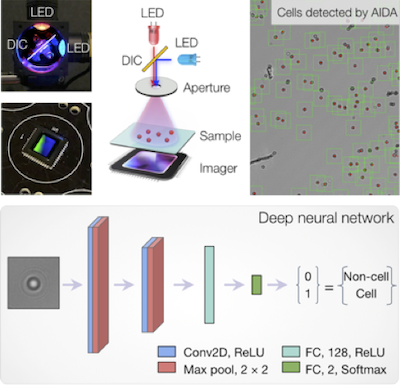
Digital sensors and improvements in computation hardware have introduced a new era of computational optics. This technology can overcome fundamental limits in conventional optics by acquiring optically-coded images that can be numerically analyzed and transformed. We aim to develop an intelligent imaging system that integrates computational optics and state-of-the-art machine vision technologies. This synergistic approach, for example, could segment and count thousands of single-cells per acquisition, automatically analyze large data sets, and extract underlying patterns beyond human recognition. Our AIDA (artificial intelligence diffraction analysis) system is the first generation prototype, which combines two core elements: i) digital holography augmented with color-based multiplexing and ii) deep-learning for automated whole-image analyses. Using fine-needle aspirate (FNA) samples from breast cancer patients, AIDA not only detected individual cancer cells, but also classified them according to their molecular profiles, producing therapeutically actionable results. Separately, for HPV detection in liquid samples, we leveraged deep learning methods to detect and count microbead dimers within hologram images. We plan to build on our deep-learning microcopy platform to include functions such as extraction of clinically meaningful single-cell feature vectors, and automated subpopulation stratification in biopsy samples.
Rapid diagnosis of infectious disease
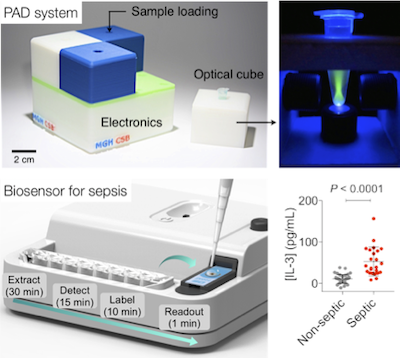
The rise of infectious diseases is a global threat to healthcare. In the US alone, more than two million people are infected with antimicrobial-resistant bacteria every year, and sepsis is responsible for more than 250,000 deaths. We seek to address these challenges in a two-pronged approach. Fast bacterial profiling. We have developed technology for bacterial detection and identification, coined PAD (polarization anisotropy diagnostics). PAD is based on a novel molecular sensing principle: changes in fluorescence anisotropy in the presence of target bacterial nucleic acids. PAD is inherently robust against environmental noise, and economically scalable for parallel measurements. This system was featured by Dr. Collins in his NIH Director’s Blog. Early detection of sepsis. We developed an integrated biosensor for rapid, onsite sepsis detection directly from blood samples. Our approach is based on emerging understanding on host response to infection: IL-3, a cytokine that activates proliferation of hematopoietic stem cells and progenitors, has been identified as a key regulator during sepsis. Our clinical study confirmed the potential of IL-3 as a sepsis diagnostic biomarker: elevated IL-3 levels were associated with high organ failure rate and greater risk of mortality. The technology is licensed to a startup company for further development.
Microfluidic systems for high precision cellular analyses
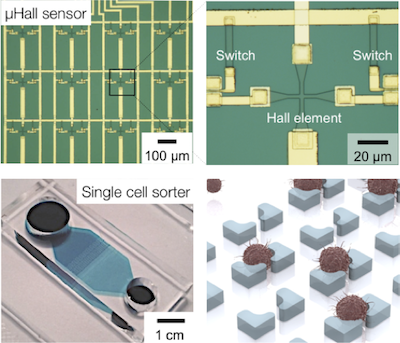
We have been advancing new microdevices to facilitate single cell studies. Our goal is to unravel previously hidden relationships between individual cells in a population. These new devices include a hybrid device combining IC (integrated circuit) chips and microfluidics to precisely control cell position; a new magnetic sorter that can enrich rare cells directly from blood; and a microfluidic chip to capture and molecular profile an array of individual cells. These systems have played an important role in translational cancer research. For instance, by applying these sensors to ovarian cancer patient samples, we isolated and molecularly profiled circulating tumor cells, identified drug-resistance states of individual tumor cells in fine-need aspirates, and probed tumor cells for a panel of markers relevant to pathway inhibition. Some of our efforts were featured in Research Highlights of the Department of Defense Ovarian Cancer Research Program.