Research Highlights

Reprogramming Tumor-associated Myeloid Immunity through MyTai
Most cancers create immunosuppressive environments dominated by tumor-associated myeloid cells that silence T-cell activity. To overcome this, our team developed called MyTai (a myeloid-targeted immune enhancer cocktail) that reprograms these cells into powerful immune activators.
In a study published in ACS Nano (2025), the team introduced a carbohydrate-based nanoparticle system that combines a refined viral RNA agonist (NexaVant, NVT) with two small-molecule immune stimulators: R848 (a TLR7/8 agonist) and LCL161 (a non-canonical NF-κB activator). Unlike lipid nanoparticles, MyTai’s cyclodextrin-based carrier avoids cationic lipids, dramatically reducing systemic toxicity while maintaining efficient delivery to tumor-associated macrophages and dendritic cells.

Personalized Blood Count Ranges for Precision Medicine
The complete blood count (CBC) is an important screening tool for healthy adults, but results are usually interpreted relative to one-size-fits-all reference intervals, undermining the precision medicine goal to tailor care for patients on the basis of their unique characteristics. CSB researchers studied thousands of patients at MGH and found that routine CBC indices fluctuate around stable values or setpoints, and setpoints are patient-specific, with the typical healthy adult’s setpoints distinguishable from those of 98% of other healthy adults, and these differences persist for at least 20 years. Setpoints in apparently healthy adults were associated with significant variation in clinical risk. Setpoints also define patient-specific reference intervals and personalize the interpretation of subsequent test results. This study shows CBC setpoints are sufficiently stable and patient-specific to help realize the promise of precision medicine for healthy adults.

Closer look into EV mRNA through SCOPE
Extracellular vesicles (EVs) in blood samples can provide valuable information about cancer heterogeneity and progression without invasive procedures. EV mRNA is particularly useful, offering insights into mutations, drug resistance, and recurrence. However, detecting EV mRNA has been challenging due to its scarcity in clinical samples. CSB researchers have developed SCOPE (Self-amplified and CRISPR-aided Operation to Profile EVs) to address this issue. By innovatively using CRISPR technology, SCOPE can detect single-base mutations in just a drop of blood. In early clinical studies, SCOPE successfully monitored tumor recurrence in colorectal cancer patients and classified glioblastoma patients. This technology could accelerate clinical decision-making and improve the use of EVs in personalized cancer treatment.

CreDiT to the Rescue: CRISPR and Digital Signal Processing for On-Site Nucleic Acid Detection
CRISPR technique has emerged as a powerful tool in nucleic acid (NA) detection. This study presents CreDiT (CRISPR Enhanced Digital Testing), a novel diagnostic system for rapid, on-site nucleic acid (NA) detection. CreDiT leverages CRISPR-Cas mediated targeting and digital signal processing inspired by digital radio communication. Specifically, the system employs digitally modulated light for sample excitation followed by numerical decoding of the measured fluorescent signal. This digital approach offers superior computational efficiency and signal-to-noise ratio compared to conventional analog methods, facilitating the development of a compact CreDiT device suitable for point-of-care applications. The researchers demonstrate the functionality of CreDiT by targeting high-risk human papillomavirus (HPV) in clinical cervical brushing specimens.
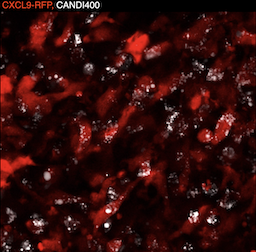
Coaxing macrophages to fight cancer
Our previous work found that high levels of tumor macrophages expressing the chemokine CXCL9 are associated with good clinical outcomes in cancer patients. Unfortunately, most tumor-associated macrophages do not express CXCL9. In this study, we develop a macrophage image screening approach to identify drugs that potentiate CXCL9 production. Here, we show a triplet drug combination incorporated into a macrophage-avid nanoparticle that strongly enhances macrophage CXCL9 production and triggers remarkable anti-tumor efficacy in mouse cancer models. We envision leveraging deep molecular analysis of anti-tumor macrophages with novel drug screening methods to develop new cancer treatment methods.
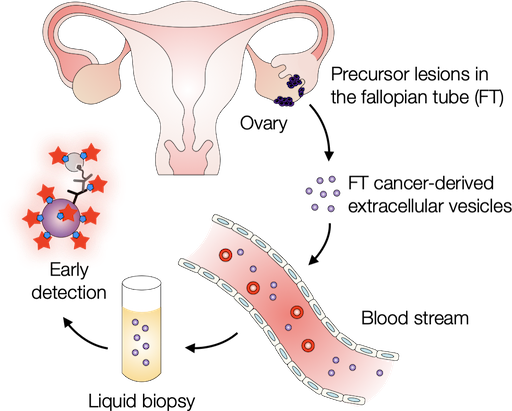
Probing extracellular vesicles promises early detection of ovarian cancer
Ovarian cancer poses challenges for early detection due to its nonspecific symptoms, often resulting in late-stage diagnosis. This study aimed to address this issue by investigating extracellular vesicles (EVs) as an early biomarker for ovarian cancer, specifically focusing on high-grade serous ovarian carcinoma (HGSOC). Leveraging the growing understanding that HGSOC originates from precursor lesions in the fallopian tube, the researchers examined EVs derived from murine fallopian tube (mFT) cells with oncogenic mutations and identified candidate markers specific to fallopian tube origins. In a pilot study involving human patients, these EV markers were validated for their high accuracy in distinguishing HGSOC from non-cancer cases. This innovative approach would offer a minimally invasive method to monitor women at high risk of ovarian cancer, facilitating timely intervention strategies.
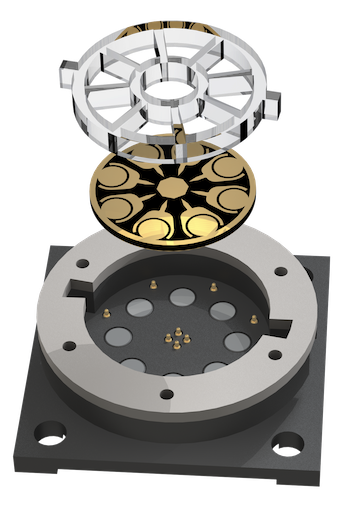
Electrical engineering to boost electrochemiluminescence
Electrochemiluminescence (ECL) is a powerful biosensing method with inherently low background and precise chemical reactions through electrical control. In recent work published in Science Advances, CSB investigators extended ECL’s advantages by devising a new system termed ECLipse (ECL in paired signal electrode). The key innovation was to separate ECL generation from target detection: these two processes were carried out in isolated chambers and coupled through an electrode. With this strategy, the investigators could maximize the analytical signal without crosstalk and integrate multiple sensors in a compact chip. In a pilot clinical study, the ECLipse system accurately detected septic conditions by measuring host factors (IL-3, IL-6, PCT) and revealed distinct IL-3 and IL-6 patterns in patients with SARS-CoV-2 infection.

Visualizing living cells & tissues in many colors
Tracking the dynamic interactions of living cells and tissues is central to our understanding of biological function, yet hard to do, because microscopic molecular machinery is intrinsically difficult to visualize while in operation. We can observe living cells/tissues by labeling key molecules with distinct fluorescent colors, but usually just one or two at a time— akin to taking photographs of a brilliant garden, but only in black and white. Other existing technologies can map the intricate spatial distribution of biomolecules and cell types within tissues (i.e., seeing the garden in all its colors), but not in specimens that remain alive and intact. Therefore, a key goal has been to achieve many-colored longitudinal readouts of living systems. Publishing in Nature Biotechnology, a CSB team introduces scission-accelerated fluorophore exchange (SAFE): imaging tools that enable living cells and tissues to be deeply and serially profiled, revealing their many-colored complexity across both space and time.

Liquid biopsy for pancreatic cancer
Pancreatic cancers are often hard to detect until it is too late. One of the reasons is that there are no sensitive or specific blood tests for early pancreatic cancer. Researchers from the Weissleder lab describe a new liquid biopsy method (sEVA) in Science Advances. The technique accurately tests for tumor shed vesicles by analyzing every vesicle in a blood sample. Preliminary feasibility studies showed that sEVA detected 15/16 stage 1 pancreatic cancer at MGH. The single vesicle detection method has the potential to transform early pancreatic cancer research and clinical practice.

ZiPPy
Minimizing nonspecific adsorption of proteins is crucial to biosensing for accurate diagnostics. In a recent paper published in Advanced Materials, the biomedical engineering team at CSB describes a new zwitterionic (‘oppositely charged’) molecule, ZiPPy (zwitterionic polypyrrole), as a superior coating material for biosensors. ZiPPy can be easily deposited onto a sensor surface through electroplating, rendering the sensor highly resistant to nonspecific protein binding. In the current study, the CSB. investigators used the ZiPPy-coated sensors to detect neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from saliva samples collected from vaccinated and unvaccinated individuals. The approach enabled antibody detection without need for sample purification or additional labeling. ZiPPy would be of great interest for the broad biosensing community.

Five-minute marijuana test
Smoking marijuana impairs cognitive/motor functions and increases the risk of vehicular accidents. Quantifying recent marijuana use remains difficult due to the low sensitivity (binary results) of rapid kits, and long turn-around of laboratory-based tests. In the paper published in Science Translational Medicine, CSB investigators describe a new sensor, EPOCH (express probe for on-site cannabis inhalation), for on-site marijuana testing. EPOCH integrates novel engineering features such as a radial membrane for molecular capture and transmission optics-based signal processing, all contained in a compact cartridge. The sensor quantifies tetrahydrocannabinol (THC), marijuana’s main psychoactive substance, at the level below the regulatory guideline within 5 minutes. In a pilot field testing, EPOCH identified recent (<12 hours) marijuana users by detecting THC in saliva samples.

Powerful new test allows a glimpse into tumor dynamics
In a recent study published in Clinical Cancer Research, CSB researchers developed a multiplexed fine needle technology to aspirate cells directly from solid tumors without the need for more invasive core biopsies or surgical excisions. The FAST-FNA method facilitates the comprehensive and quantitative assessment of cellular and functional immune biomarkers within the tumor microenvironment at the single-cell level and in situations when the samples are too scant to perform flow cytometry. Serial immune profiling is an advancement in the field in both concept and methodology that allows for the early detection of changes induced within the tumor microenvironment by various applied therapeutics, providing a platform for the development and discovery of quantitative multi-dimensional scores and/or biomarkers that may allow for improved and timely prediction of treatment response.

A molecule that may help prevent Alzheimer’s disease
Communication within the glial cell ecosystem is essential for neuronal and brain health. We found, in humans and mice, that astrocyte-sourced interleukin-3 (IL-3) programs microglia to ameliorate the pathology of Alzheimer’s disease (AD). Upon recognition of β-amyloid (Aβ) deposits, microglia increase their expression of IL-3Rα—the specific receptor for IL-3—making them responsive to IL-3. Astrocytes constitutively produce IL-3, which elicits transcriptional, morphological, and functional programming of microglia to endow them with an acute immune response program, enhanced motility, and the capacity to cluster and clear aggregates of Aβ and tau. These changes restrict AD pathology and cognitive decline. Our findings identify IL-3 as a key mediator of astrocyte–microglia cross-talk and a node for therapeutic intervention in AD.

Cancer: immunotherapies without side effects?
Immune checkpoint blockade (ICB) has revolutionized cancer therapeutics; however, in many cases, ICB is limited by immune-related adverse events (irAEs). Thus, a better understanding of the immune responses that lead to irAEs and how they are distinguished from antitumor immunity is needed. Here, Siwicki et al. used anti-CD40 therapy as a mediator of TH1-induced antitumor immunity in mouse tumor models. They found that liver-resident Kupffer cells induced neutrophil-mediated liver toxicity by producing IL-12 and responding to IFN-γ. Inhibition of the neutrophil response limited liver toxicity while retaining the antitumor efficacy of anti-CD40. Similar data were found in patients treated with anti–PD-1 and anti–CTLA-4. Together, these data suggest that the toxicity of ICB can be inhibited without negatively affecting antitumor immunity.

Detecting and monitoring colorectal cancer in blood
In a new study published in Nature Biomedical Engineering, CSB investigators report a new high-throughput assay system for extracellular vesicles (EVs). Termed HiMEX (high-throughput integrated magneto-electrochemical extracellular vesicle), the system integrates EV enrichment and sensing in a single assay, and carries out parallel measurements in a 96-well plate format. Using plasma samples from patients with colorectal cancer (CRC) and healthy volunteers, the investigators identified a panel of EV biomarkers for CRC detection (96% accuracy). In a prospective cohort, EV profiles enabled assigning patients to a high- or a low-risk 5-year disease-free survival group, and monitoring tumor relapse
during therapy.

A spoonful of albumin helps the medicine go down
Albumin has long been used to improve drug circulation and targeting in the body. In principle, this concept has been attractive for treating tumors with appetites for protein nutrients that fuel malignant growth. Nonetheless, albumin-based delivery mechanisms in cancer remain difficult to understand. In a recent Nature Nanotechnology report, an MGH-CSB team demonstrated how oncogenic KRAS signaling controls albumin-drug uptake in mouse tumor models. The team discovered that therapeutically manipulating nutrient signaling through inhibition of insulin-like growth factor 1 receptor (IGF1Ri) could enhance tumor consumption and efficacy of the albumin-formulated chemotherapeutic, nab-paclitaxel. These results offer new possibilities to improve the selective delivery of albumin-binding drugs in patients.

Cycling between life and death
Small molecule MDM2inhibitor changes the temporal p53 expression in tissues and can render them more radiosensitive. This has potentially important applications in radiation oncology. The study published in Nature communications by the Lahav and Weissleder teams shed new light on the temporal p53 expression in tissues and proposes a new paradigm on exploiting differences in p53 expression levels.
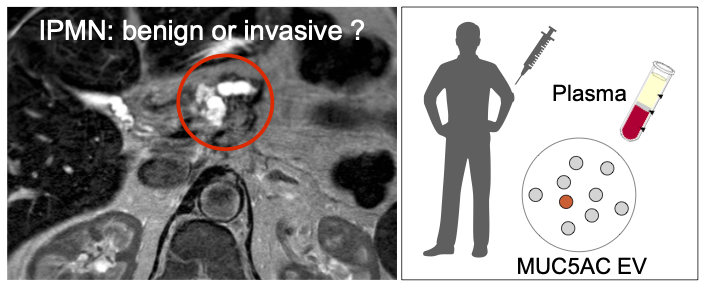
Identifying high grade dysplasia and invasive early pancreatic cancers may just have become easier
Increasing detection of intraductal papillary mucinous neoplasms (IPMNs), cystic tumors of the pancreas, from cross-sectional imaging is a problem for clinicians since these patients will require prolonged surveillance. A non-invasive method for the distinction of benign from invasive IPMNs is an unmet clinical need. The authors from the Center for Systems Biology, Surgery, Radiology and Pathology developed a blood based digital extracellular vesicle (EV) screening technology (DEST) that permits the distinction of invasive IPMNs from low grade and non-invasive subtypes. In a study of 133 patients, MUC5AC EV profiling reliably identifies patients with invasive IPMN. When combined with imaging and clinical findings, the DEST method has the potential to transform IPMN/early PDAC cancer detection and surgical evaluation, including avoiding unnecessary surgeries.

Routine blood test may predict COVID-19 hospital death risk
Coronavirus disease 2019 (COVID-19) is an acute respiratory illness with a high rate of hospitalization and mortality. Biomarkers are urgently needed for patient risk stratification. In a recent paper in JAMA Network Open, a team of investigators at the MGH Center for Systems Biology has reported that a standard test that assesses variations in red blood cell volume (RDW) can identify hospitalized patients with COVID-19 at the time of admission who have a 2.7x increased risk of mortality. Patients who had RDW values above the normal range when they were admitted to the hospital had a mortality rate of 31 percent compared with 11 percent in patients with normal RDW values. An increasing RDW during hospitalization was also associated with increased mortality.

New breakthrough for ultrafast bed-side cancer diagnosis
Rapid, automated, and point-of-care cellular diagnosis of cancer remains difficult in resource-limited settings due to lack of specialists and medical infrastructure. In a recent paper published in Science Translational Medicine, the biomedical engineering team at the Center for Systems Biology has developed an automated image cytometry system (CytoPAN) that allows rapid breast cancer diagnosis and receptor subtyping in 1 hour using as few as 50 cells obtained by fine needle aspiration (FNA). The combination of FNA and CytoPAN offers an alternative strategy for faster, minimally invasive cancer diagnosis in both developed and developing countries. Coupled with recently developed cycling technologies for FNA, this will also enable rapid molecular and cellular profiling of serial tumor samples in clinical trials.

All metastases are not created equal
Metastases can form in locoregional lymph nodes – a form of progression that portends a worse prognosis but can still be curable – or they can develop in distant organs. Treatments for the latter case are typically considered palliative. It is unknown whether lymph node and distant metastases are only distinguished by their different prognostic implications, or whether the biology underlying their formation is also distinct.
In a new study, published in Nature Genetics, the Naxerova lab at the Center for Systems Biology and collaborators at the Canary Center for Cancer Early Detection at Stanford now show that lymph node and distant metastases develop through different evolutionary mechanisms. Reconstructing the evolutionary histories of dozens of colorectal cancers, the team showed that lymph node metastases are a genetically highly diverse group. Their pronounced heterogeneity indicates that they can be seeded by many different primary tumor sub-lineages. In contrast, distant metastases are homogeneous. They typically resemble each other and have a recent common ancestor, suggesting that fewer primary tumor subclones possess the ability to form lesions in distant organs. These results show that the selective pressures shaping metastasis development in different anatomical sites differ substantially.
Breaking bad habits in cancer therapy
Targeted cancer drugs are designed to kill tumor cells, but can elicit a tumor-protective wound-healing response as the body repairs its dying tissue. In a report published in Science Advances, a team at the MGH Center for Systems Biology and the Massachusetts Institute of Technology collaborated to understand mechanisms of how the immune response to kinase inhibitors could promote drug resistance often seen in patients. Using a computational pipeline to interpret multicellular signaling in the tumors of patients with melanoma or ovarian cancer, the team found that tumor cells adopted a program of wound healing especially related to the role of macrophages in clearing debris from dying cells. This signaling co-amplified between tumor cells and neighboring macrophages to cause drug resistance in a reinforcing feedback loop. To break this destructive cycle, the team formulated a nanotherapy that efficiently accumulated in the phagocytic macrophages of resistant tumors and delivered a toxic payload that blocked resistance.

CSB work discovers exercise benefits on bone marrow
Modern life comes with many dangers. This includes sedentary behavior, as we all spend increasing time sitting: on the office chair, in the car, and on the sofa. This is risky and leads to obesity, myocardial infarction and stroke. A manuscript published in Nature Medicine describes a new pathway how sedentary behavior causes cardiovascular disease. Mice with access to treadmills had lower blood stem cell proliferation in their bone marrow. On the flip side, sedentary mice, resembling a large proportion of the American population, work out 20-fold less. As a result, they had higher blood levels of the hormone leptin, which triggered over-production of inflammatory leukocytes, resulting in more atherosclerosis and heart failure. The team also looked at clinical data from the CANTOS trial, observing similar relationships between physical activity, leptin and leukocytes.

Discovering Myeloid Cells In Lung Tumors
Myeloid cells can promote or limit tumor outgrowth but remain poorly understood. In a study published in Immunity, the Pittet lab at the MGH Center for Systems Biology and the Klein lab at Harvard Medical School teamed up to map myeloid cells at the single cell level in human and mouse lung cancer. They made the following findings: 1) Consistent complexity: the same tumor myeloid populations are repeatedly found across patients, indicating that the myeloid microenvironment within lung tumors is stereotyped. 2) Conservation across species: many myeloid populations are highly conserved across patients and mice, suggesting that studying myeloid cells in mice can help understand the human disease. 3) New therapeutic targets: the map of tumors’ myeloid populations identified in this study points to new targets for cancer immunotherapy.