Hulsmans Lab
Read BioThe Hulsmans lab focuses on the system-wide interrogation of the cardiac conduction system to understand its function at the molecular level, and to identify new drug targets.
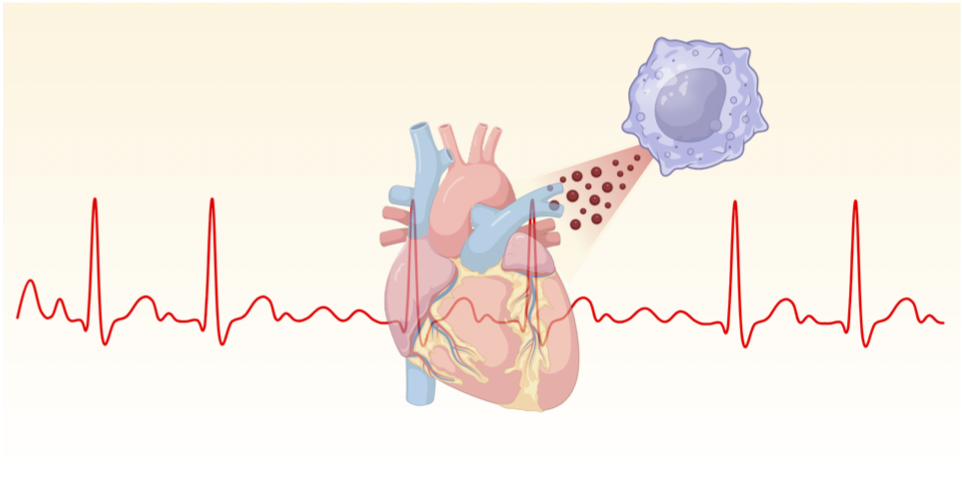
The conduction system is composed of a heterogeneous population of cells including cardiomyocytes, fibroblasts, endothelial cells, vascular smooth muscle cells, pericytes and immune cells. Cardiac macrophages, the most abundant immune cell in the healthy heart, is an immediate focus of the lab due to their diverse phenotypes and functions, and their ability to quickly adapt to the tissue environment and changes therein.
It is now clear that intercellular signaling and cross-talk between cardiomyocytes and non-cardiomyocytes including macrophages are critical in the initiation and propagation of cardiac remodeling. Such remodeling processes follow different types of cardiac stress, like myocardial infarction, myocarditis, valve disease or chronic hypertension, and when uncontrolled can lead to heart failure or cardiac arrest resulting from pulseless electrical activity or arrhythmia. Yet, the complex network of intercellular circuits of communication is poorly understood.
By employing innovative mouse models of arrhythmia along with state-of-the-art multi-omics technologies, the Hulsmans lab aims to unravel the complex biology linking macrophage heterogeneity to adverse cardiac remodeling and arrhythmia.
Recent Publications
Grune J, Bajpai G, Ocak PT, Kaufmann E, Mentkowksi K, Pabel S, Kumowski N, Pulous FE, Tran KA, Rohde D, Zhang S, Iwamoto Y, Wojtkiewicz GR, Vinegoni C, Green U, Swirski FK, Stone JR, Lennerz JK, Divangahi M, Hulsmans M, Nahrendorf M Virus-Induced Acute Respiratory Distress Syndrome Causes Cardiomyopathy Through Eliciting Inflammatory Responses in the Heart. Circulation. 2024;:ePub - PMID: 38506045 - DOI: 10.1161/CIRCULATIONAHA.123.066433
Zhang S, Paccalet A, Rohde D, Cremer S, Hulsmans M, Lee IH, Mentkowski K, Grune J, Schloss MJ, Honold L, Iwamoto Y, Zheng Y, Bredella MA, Buckless C, Ghoshhajra B, Thondapu V, van der Laan AM, Piek JJ, Niessen HWM, Pallante F, Carnevale R, Perrotta S, Carnevale D, Iborra-Egea O, Muñoz-Guijosa C, Galvez-Monton C, Bayes-Genis A, Vidoudez C, Trauger SA, Scadden D, Swirski FK, Moskowitz MA, Naxerova K, Nahrendorf M Bone marrow adipocytes fuel emergency hematopoiesis after myocardial infarction. Nat Cardiovasc Res. 2023;2(12):1277-1290 - PMID: 38344689 - PMCID: PMC10857823 - DOI: 10.1038/s44161-023-00388-7
Hulsmans M, Schloss MJ, Lee IH, Bapat A, Iwamoto Y, Vinegoni C, Paccalet A, Yamazoe M, Grune J, Pabel S, Momin N, Seung H, Kumowski N, Pulous FE, Keller D, Bening C, Green U, Lennerz JK, Mitchell RN, Lewis A, Casadei B, Iborra-Egea O, Bayes-Genis A, Sossalla S, Ong CS, Pierson RN, Aster JC, Rohde D, Wojtkiewicz GR, Weissleder R, Swirski FK, Tellides G, Tolis G, Melnitchouk S, Milan DJ, Ellinor PT, Naxerova K, Nahrendorf M Recruited macrophages elicit atrial fibrillation. Science. 2023;381(6654):231-239 - PMID: 37440641 - PMCID: PMC10448807 - DOI: 10.1126/science.abq3061
Bapat A, Schloss MJ, Yamazoe M, Grune J, Hulsmans M, Milan DJ, Nahrendorf M, Ellinor PT A Mouse Model of Atrial Fibrillation in Sepsis. Circulation. 2023;147(13):1047-1049 - PMID: 36972346 - PMCID: PMC10057612 - DOI: 10.1161/CIRCULATIONAHA.122.060317
Grune J, Lewis AJM, Yamazoe M, Hulsmans M, Rohde D, Xiao L, Zhang S, Ott C, Calcagno DM, Zhou Y, Timm K, Shanmuganathan M, Pulous FE, Schloss MJ, Foy BH, Capen D, Vinegoni C, Wojtkiewicz GR, Iwamoto I, Grune T, Brown D, Higgins J, Ferreira VM, Herring N, Channon KM, Neubauer S, Oxford Acute Myocardial Infarction (OxAMI) Study, Sosnovik DE, Milan DJ, Swirski FK, King KR, Aguirre AD, Ellinor PT, Nahrendorf M Neutrophils incite and macrophages avert electrical storm after myocardial infarction. Nat Cardiovasc Res. 2022;1(7):649-664 - PMID: 36034743 - PMCID: PMC9410341 - DOI: 10.1038/s44161-022-00094-w
- More publications ...