Newly discovered cell revs up immune system in sepsis
All doctors, especially those in intensive care, dread the sudden change in temperature, clammy skin and drop in blood pressure that hallmark the onset of sepsis—a condition that can lead to multi-organ failure and death. Imagine then if on admission to hospital, patients could undergo a minor intervention to boost their resistance to infection and dramatically reduce their chances of sepsis. This possibility may not be so far fetched in light of the recent finding of an important, but hitherto undiscovered, immune cell that upon encountering infection in the body sounds a “molecular alarm”, which has the effect of activating other immune cells to be more efficient at clearing bacteria. In theory, by boosting the action of this cell, it could be possible to rally up the body’s immune system to better overcome infection. Researchers at the Center for Systems Biology (CSB) report their findings in the February issue of Science.
In the US, more than 210,000 people develop sepsis each year, a lethal condition with a high mortality rate. While these numbers are concerning enough, these figures are rising, in most part due to increasing antibiotic resistance. As a result, alternative strategies are now being actively sought in an attempt to prevent patients from entering into this dangerous state. “Once a patient goes into sepsis there’s really very little you can do,” explains Filip Swirski, Assistant Professor at the CSB and senior author of the study. “It goes very fast. The patient usually dies within a day or two.”
To date, because of its immunologically complex pathophysiology, sepsis has remained a therapeutic challenge. On the one hand, it is characterized by overwhelming infection, which the body tries to fight; but on the other hand, an overzealous immune response can lead to dangerous inflammation. Left unchecked, this can result in multi-organ damage and ultimately death. The therapeutic goal for sepsis is therefore to strike a balance between controlling infection and controlling inflammation.
One way of achieving this balance could be to boost the immune system somehow such that it is more effective at getting rid of bacteria. And for this, targeting the newly discovered immune cell—dubbed ‘innate response activator’ (or IRA)—could be precisely the answer.
Surprising cell
The discovery of IRA cells was an accident. The research team led by Filip Swirski stumbled upon these cells whilst investigating an entirely different problem. Namely, they were looking at growth factors in the spleen, following up on a previous discovery which highlighted this organ as an important reservoir for monocytes—a white blood cell with essential immune functions. It was thus while examining growth factors in the spleen involved in the production of white blood cells that the team discovered the presence of GM-CSF (short for granulocyte macrophage-colony stimulating factor), an elusive protein previously implicated in blood cell production. While the presence of this factor in the spleen was not surprising per se, the source of this protein turned out to wholly unexpected. “It had always been thought that GM-CSF is predominantly made by non-blood cells,” explains Swirski, “or if it is made by blood cells, that it would be made by macrophages.” However, to the researchers surprise, the GM-CSF-producing cells in the spleen were identified as members of the B cell family, a white blood cell type known for its production of antibodies. “We definitely didn’t expect that GM-CSF would be made by B cells,” confesses Swirski.
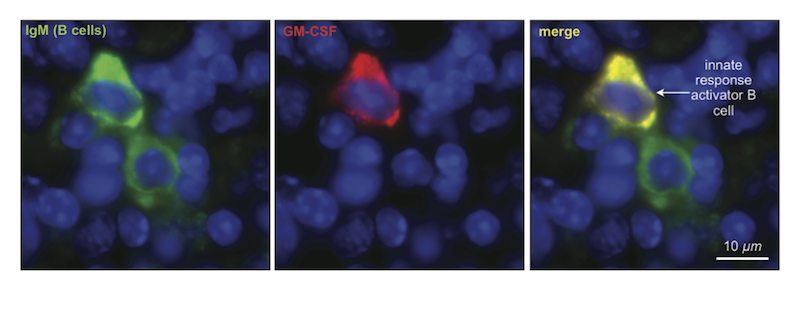
IRA cells, however, were discovered as a unique population of B cell. That is, while they looked like other B cells in terms of their surface markers, their gene expression and ultimately their function was very different. The biggest difference was that in addition to producing antibodies, IRA B cells also have a separate system that works in parallel to produce GM-CSF.
Having discovered this new immune cell, the investigators were subsequently interested to figure out where these cells fit in, with regards the immune system’s hierarchy of response. Their initial assumption was that, like conventional B cells, these cells formed part of the so-called ‘adaptive’ immune response or second wave of immunity. But once again, the researchers were surprised by their findings. IRA B cells were actually identified as members of the B1 cell family, a group implicated in innate immunity, which is the body’s first line of defense. “This finding was critical,” explains Swirski, “as it suggests that the accepted hierarchy of an immune response may not be complete.”
In view of their novel discovery, the researchers were then curious to know what role this cell plays during an immune response. To find out, they developed a genetically engineered mouse in which they “knocked-out” the capacity for B cells to make GM-CSF; they then induced sepsis. In so doing, they discovered that mice without this functionwere unable to mount a defense against sepsis and thus died much earlier and in greater numbers than normal animals. On subsequent examination of the inflammatory markers in mice lacking IRA B cells, it appeared that their poorer outcome was due to deficient bacterial clearance.
Emergency messenger?
According to Swirski, IRA B cells likely function as an alarm to the immune system: whereupon detecting sites of infection, these cells travel to the spleen, an organ known to contain cells that are important for the recognition and clearance of bacteria. “You could think of it as an ‘emergency messenger’,” says Swirski. Namely, by traveling to and releasing GM-CSF in the spleen, the immune system is called into action and somehow renders immune cells better equipped to fight bacteria.
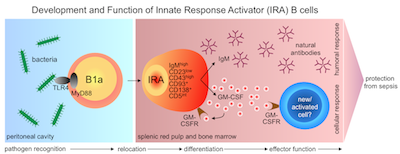
The discovery of these cells is pivotal as they now open up new avenues for clinical research. As already outlined, GM-CSF could be targeted for therapeutic benefit to boost patients’ immune systems prior to surgery. Another possible use for IRA B cells could be as biomarkers for disease since their numbers have been found to vary in different patient populations.
“In order to move forward, however, we need to understand the biology of IRA B cells in more detail,” says Swirski. The research team are currently looking into the role of IRA B cells in other infectious models and in other inflammatory diseases.
Written by Yvonna Fisher-Jeffes, PhD
Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK
Innate Response Activator B Cells Protect Against Microbial Sepsis
Science. 2012;335(6068):597-601 – PMID: 22245738 – PMCID: PMC3279743
Press Coverage
ScienceNOW – Surprising Cells Stymie Sepsis (pdf)
Nature Reviews Immunology – B cells: Protective role of innate-like B cells in sepsis (pdf)
Mass General Hospital – Newly identified type of immune cell may be important protector against sepsis (pdf)
Nature Immunology – Research Highlight (pdf)
Science Signaling Editor’s Choice – Immune Sentinels (pdf)
MedicalXpress, USA Today, Science Newsline
New Scientist – New bug-fighter cell may force immune response rethink (pdf)
Jackson Labs Newscast – Gaining in the Battle Against Sepsis (pdf)
Jackson Labs Podcast (mp3)
Faculty 1000
Immunotherapy – Future Medicine (pdf)